[Unpublished manuscript; new taxonomic names are invalid and should not be used in scientific publications.]
Also see: AXSMITH, BRIAN J.*, THOMAS N. TAYLOR, AND EDITH L. TAYLOR. Department of Botany, University of Kansas, Lawrence, KS 66045. - A new cheirolepidiaceous conifer from the Lower Jurassic of North America.
A NEW CHEIROLEPIDACEOUS CONIFER BEARING FLOWERS FROM THE EARLY JURASSIC OF CONNECTICUT, USA
Bruce Cornet1 and Nicholas G. McDonald2
(Manuscript written in 1995, presented by B. Cornet at the GSA Convention in Cromwell, CT, on 21 March 1995)
1 27 Tower Hill Ave., Red Bank, NJ 07701
2 Dept. of Earth and Environmental Sciences, Wesleyan University, Middletown, CT 0645
Abstract
Two new cheirolepidaceous conifer taxa belonging to the new subfamily Conanthoideae (defined) are described from the lower middle Portland Formation of the Hartford basin, Connecticut. Both taxa come from the same red and gray lacustrine strata at the K-F quarry locality in Suffield, CT, which is the youngest fossil plant locality in the Newark Supergroup. Its age is late Sinemurian-early Pliensbachian based on Milankovich cyclostratigraphy, palynology, and insect fossils. Both taxa are described as having a new type of dwarf-shoot that possesses ten distal lobes arranged in two rows. One taxon, Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov. (natural genus for entire plant), is in organic attachment with bracts, cones, and branch systems. The bract is unusual in being long, narrow, and sagittate, while the lobes on the dwarf-shoot are extremely long (up to 17 mm). The second taxon is based on dispersed scales. It is placed in Conanthopsis hartfordensis Cornet and McDonald gen. et sp. nov. (form-genus for dispersed scales). A complete maturation series of male and female cones demonstrates a flower-like early stage followed by a cone-like seed dispersal stage, where the cone axis does not initially elongate and the ovuliferous scales do not completely develop until after pollination. Evidence is presented for an angiosperm-like reproductive strategy involving pollen that germinates on paired hollow stigma-like lobes on the dwarf-shoot and an ovule that is fertilized via pollen tubes in an ovary. Pollen extracted from attached and unattached male cones is of the Corollina torosa type. A leafy shoot possibly belonging to C. hartfordensis is placed in Pagiophyllum simile (Newberry) Cornet and McDonald comb. nov. One additional new combination is made: Hirmeriella estonensis (Kendall) Cornet and McDonald comb. nov. Evolutionary innovations and trends of cheirolepidaceous conifers in the Newark Early Jurassic are discussed.
Introduction
The family name, Cheirolepidiaceae, was originally established by Hirmer and Hörhammer (1934) for the genus, "Cheirolepis" Schimper, which is a later illegitimate pseudonym of a genus in the Asteraceae. Jung (1968) recognized this nomenclatoral problem, but also recognized that associated cone scales placed under the name, Hirmeriella, were the bracts to the ovuliferous scales called "Cheirolepis". Jung (1968) also notes that Saporta was perhaps the first person to realize this relationship, a relationship not recognized by Hirmer and Hörhammer (1934). Subsequently, Jung (1968) combined both types of cone scales under the name, Hirmerella, and made this taxon the type for the family. Jung shortened the name from Hirmeriella by removing the "i" to make it more euphonious. This transformation was followed by Harris (1979), but rejected by most other authors (Taylor, 1981; Watson, 1982; Van Konijnenburg-Van Cittert, 1987; Meyen, 1987; Watson, 1988; Clement-Westerhof and Van Konijenburg-Van Cittert, 1991; Taylor and Taylor, 1993). Harris (1979) also used the name Hirmerellaceae for the family instead of Cheirolepidiaceae. However, the family name Cheirolepidaceae has been published legitimately, and therefore should be used. Clement-Westerhof and Van Konijnenburg-Van Cittert (1991) refer to the ovuliferous scale as a dwarf-shoot, and distinguish between fertile and sterile lobes on the dwarf-shoot, conforming to terminology used for Paleozoic conifers. Because of confusion created by additional lobes and their specialized functions in Conanthus, the terms fertile and sterile will not be used in this paper. Instead, dwarf-shoot scales will be referred to by the row in which they occur. Since there are only two rows, the row closest to the dwarf-shoot axis is referred to as the adaxial row, and the row furthest from the axis is called the abaxial row.
Subsequent to this early work, similar types of ovuliferous scales (dwarf-shoots) belonging to this family have been described infrequently. Most have been found unattached and associated with vegetative shoots: e.g. with Pagiophyllum araucarinum (Late Liassic- Bajocian), P. maculosum (Bajocian), Frenelopsis oligostomata (Senonian), and Pseudofrenelopsis parceramosa (Berriasian-Albian)(Van Konijnenburg-Van Cittert, 1987). Only a couple cone scales have been assigned to the genus Hirmeriella: i.e. H. estonensis (Kendall) nov. comb., which is associated with and has the same type of cuticle as P. araucarinum (Van Konijnenburg-Van Cittert, 1987), and P. kendalliae, which is associated with and has the same type of cuticle as P. maculosum (Harris, 1979). Similar types of cones and dwarf-shoots were found attached to Early Cretaceous leafy shoots belonging to the genus, Tomaxellia (Archangelsky, 1968), in particular the species T. biforme. Unnamed Hirmeriella-like dwarf-shoots have also been found associated with Brachyphyllum scottii (early Liassic Newark Supergroup: Cornet, 1977 and here), associated with Brachyphyllum crucis (Bajocian-Callovian: Van Konijnenburg-Van Cittert, 1987), and associated with various leafy shoot taxa in Early Jurassic rocks of the Newark Supergroup (Cornet et al., 1973; Cornet, 1977; Olsen et al., 1992).
Male cones have been found attached to and associated with a wide variety of cheirolepidaceous shoots. They are typically placed in the genus Classostrobus if they contain the uniquely characteristic pollen of the family, Corollina (= Classopollis), and if they are not attached. The male cones are usually round to oblong (elliptical to globose: Barnard, 1968; Van Konijnenburg-Van Cittert, 1971), and vary considerably in size from as small as 2-3 mm long and 1 mm wide Tomaxellia biforme) to as large as 23 mm in diameter (Suturovagina intermedia). Both extremes in size come from the Cretaceous (Van Konijnenburg-Van Cittert, 1987). Most male cones from the Newark Supergroup (early Liassic) range from 7 mm in diameter to as large as 12 mm long and 10 mm wide, but the cones of the new species described here range up to 20 mm in diameter. Very few male cones are found attached. Mostly, they occur associated with the vegetative shoots to which they belong. If only one type of vegetative shoot occurs with the cone, a relationship is commonly suspected or assumed if bract cuticles match those of the leaves. Only Hirmeriella muensteri, Brachyphyllum crucis, T. biforme, and the new taxon have been found with attached male cones. In each of these examples female cones have also been found attached (Van Konijnenburg-Van Cittert, 1987).
Pollen morphology is perhaps the most uniform character of the family (Taylor and Taylor, 1993). It also appears to have been one of the most conservative organs throughout the long range of this group (Late Triassic to Cretaceous). Various names have been applied to this pollen, including Classopollis, Circulina, Corollina, and Gliscopollis. There has been considerable controversy over which of these names has priority and which should be used (e.g. Boltenhagen, 1973; Traverse et al., 1975; Cornet and Traverse, 1975; Srivastava, 1976; Medus, 1977; Traverse, 1988). Selection of one name has been considered subjective (Srivastava, 1976; Traverse, 1988), based on how one regards and treats Malyavkina's (1949) line drawings. Those who concluded that they are inadequate for determining identity use Classopollis, while those who regard her drawings as illustrations of circumpolloid taxa (there are no other drawings in her paper that compare with such morphotypes, which are present in abundance in the rocks she sampled), use Corollina (or Corollina and Circulina) instead of Classopollis (Traverse, 1988). It is interesting that the taxonomic name used most frequently for papers dealing with Late Jurassic and Cretaceous sediments is Classopollis, while that most frequently used name for Triassic and Early Jurassic sediments is Corollina. The division appears to be more along time lines and convention rather than rules and logic. In this paper I recognize and use Corollina as the name having priority (see Cornet and Traverse, 1975).
Pollen cones containing either Corollina meyeriana or C. torosa have been found, along with cones containing a mixture of up to three different dispersed Corollina taxa (see below; Cornet, 1977; Cornet and Traverse, 1975). No circumpolloid pollen taxa restricted to the Triassic have been recovered from pollen cones. It is not until the Early Jurassic that the Cheirolepidiaceae become dominant in world floras, and their cones become common. The oldest cheirolepidaceous female cones in the Newark Supergroup, for example, come from basal Jurassic strata in the Newark basin (near Clifton, NJ). The oldest female cones in Europe (Hirmeriella muensteri) also come from basal Jurassic (i.e. "Rhaeto-Liassic") and early Liassic strata of Bayreuth, Nurnberg, and "Sandgrube Lautner, Unternschreez", West Germany (Jung, 1968; Watson, 1988; Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991).
Corollina meyeriana is the oldest recognized pollen type of this family, ranging back to the Carnian (early Late Triassic) in the Newark Supergroup (Cornet, 1993). Other morphologically related types, such as Duplicisporites, Praecirculina, Paracirculina, and Partitisporites, which evolved at a time when this group was undergoing its initial radiation (Traverse, 1988), may belong to sister taxa within the order, Cheirolepidales. Intrastriate- intrabaculate morphotypes, usually typified by the species, C. torosa, do not appear until the latest Norian/earliest Rhaetian (Morbey, 1975; Cornet, 1993). An unusual circumpolloid pollen taxon with gemmate sculpture first appears along with C. torosa at the beginning of the Rhaetian: Granuloperculatipollis rudis, however, is restricted to the Triassic (Venkatachala and Goçzan, 1964). Corollina taxa possessing various types of non-gemmate sculpture (scabrate, microechinate, and macroechinate) don't begin to appear until the Hettangian, and aren't common until the Late Jurassic-Early Cretaceous (Burger, 1965; Reyre, 1970).
The exine structure of cheirolepidaceous pollen has attracted much interest because of its tectate collumellate structure (Pettitt and Chaloner, 1964; Taylor and Alvin, 1984; Medus, 1977; Taylor and Zavada, 1986). Taylor and Alvin (1984) suggest that the complex structure of the wall of more evolved Corollina taxa indicates a level of biological organization that signals the evolution and subsequent refinement of incompatability systems. In addition, there is some evidence (further advanced here) that the seeds were enclosed in a pouch that opened proximally on the scale (e.g. Jung, 1968). This would imply that pollen did not have direct access to ovule micropyles, and that the pollen may have germinated on the scale in contrast to the typical gymnospermous condition (Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991). The resemblance of pollen wall structure to that of angiosperms, pollen dispersal as facultative and sometimes obligate tetrads (or diads in some species, e.g. Dicheiropollis etruscus, Jardiné (Doerenkamp, and Biens, 1974), and the cloaking or protection of the ovules are unusual gymnospermous features that could indicate the involvement of animal vectors in pollination (Hughes, 1976; Watson, 1988).
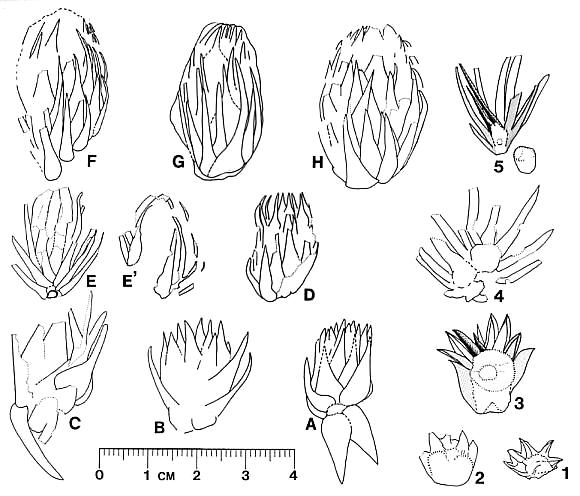
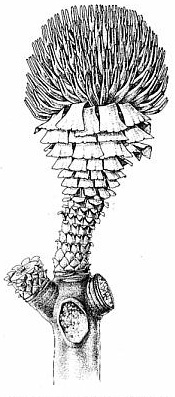
The amount of reproductive information on this family is regrettably small due to the limited number of female cone types available for study. Leaf diversity is much greater, and includes such form-genera as Androvettia, Brachyphyllum, Cupressinocladus, Elatides, Frenelopsis, Geinitzia, Glenrosa, Pagiophyllum, Pseudofrenelopsis, Suturovagina, Tarphyderma, and Tomaxellia (Alvin and Pais, 1978; Harris, 1979; Watson, 1988; Taylor and Taylor, 1993). Based on that diversity, particularly the extremes in leaf morphology that evolved in the Cretaceous, one might expect that seed and dwarf-shoot morphology would be equally diverse. Yet the dwarf-shoots of Tomaxellia from the Early Cretaceous are not significantly different from Hirmeriella muensteri. The only difference seems to be the arrangement of the six lobes on the scale: In Hirmeriella five lobes are aligned in one row with a sixth lobe in a second "row", while in Tomaxellia there is only one row containing 6 lobes. Furthermore, the bracts appear to be similar both in relative size and shape for these two taxa. Yet, Harris (1979) demonstrated that some Yorkshire Jurassic members of the family diverged considerably from this "bauplan". For example, Hirmeriella kendalliae possessed a dwarf-shoot with 10-12 lobes of unequal size and two ovules, each contained within a separate cutinized pouch or epimatium (Text-fig. 7).
Cheirolepid dominance in eastern North America
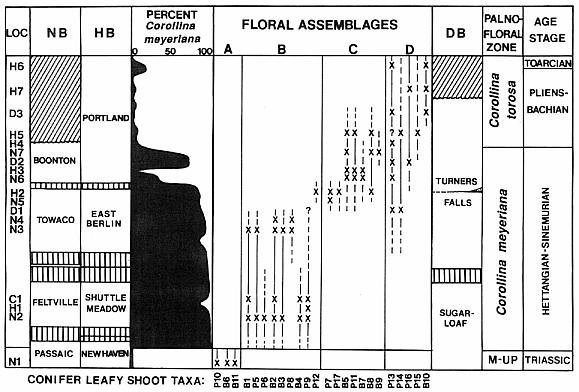
The diversity of dispersed cheirolepidaceous dwarf-shoots and leafy shoots in Hettangian-Pliensbachian-age sediments of the Hartford and Deerfield basins of Connecticut and Massachusetts, USA, is much greater than that published for other areas and periods (Text-fig. 1; see Cornet, 1977). The area encompassed by the central and southeastern parts of North America, because of its paleoequatorial tropical to subtropical location, appears to have been a hot-bed for evolution in this family. There are scales (dwarf-shoots) with similar morphology to that of Hirmeriella, but also scales that have fewer and greater numbers of lobes (as few as three and as many as ten in the Newark Supergroup). There are taxa with one seed inside the pouch formed by an epimatium or cover (the common condition), and taxa with two seeds per scale (each within its own pocket or enclosure: See Harris, 1979). But it wasn't until the discovery of the taxa described in this paper that something truly extraordinary and evolutionary could be documented for this family: A reproductive strategy analogous to that of angiosperms.
NB = Newark basin; HB = Hartford basin; DB = Deerfield basin; localities from Cornet 1977.
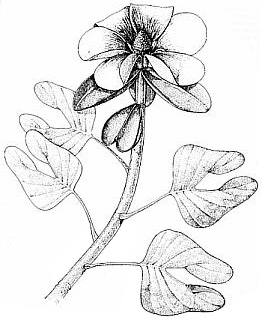
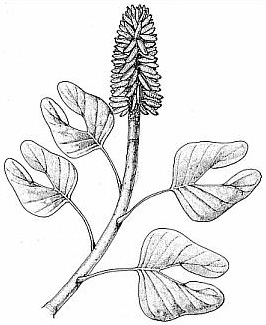
New locality (subject of this paper) is stratigraphically located above H6; its fossil flora represents Floral Assemblage E (not shown).Through the sequence of Early Jurassic sediments in the Hartford basin there is a trend in leaf size from small and short to large and long (Figs. 1-3, 4-12; Text-figs. 1, 8-9; Cornet, 1989). At the youngest fossil plant locality in the Portland Formation near the Connecticut River and Suffield, CT, the new taxa described in this paper were discovered by the junior author in late 1991. In addition to possessing some of the longest leaves yet recorded for any member of this family (compare Figs. 4 through 12), the collection contains dozens of mature and immature cones, both attached and dispersed among numerous leafy shoots with up to four branch orders. Permineralized branches and logs are also common. What is so remarkable about the cones is the number and extreme length of the lobes on mature dwarf-shoots, the round hollow vascularized condition of stigma-like lobes positioned above the micropyle of an amphitropous ovule in the pouch, and the flower-like morphology of immature cones (long sagittate petaloid bracts arranged in a rosette) prior to cone axis elongation and dwarf-shoot development.
Locality and stratigraphy
To be completed.
Photographs of fossil plant locality; N.G. McDonald and rock hammer for scale.
Environment and age based on palynofloral analysis
The palynoflorule from the gray and red lacustrine shales is strongly dominated by pollen (95.9-97.2%), as are most other palynoflorules from the Portland Formation (90+%: Cornet and Traverse, 1975; Cornet, 1977). The breakdown of the K-F quarry palynoflorule by morphotypes is given in Table I. The palynomorph assemblage is similar to that of the youngest previously studied palynoflorules at the bridge along the Westfield River in Agawam, Massachusetts (Cornet, 1977; Cornet, 1993a).
The Agawam bridge locality (AGA1-6 in Cornet, 1977) is estimated to be 1,130 meters above the Hampden basalt near the top of the lower Portland Formation. The gray to black shales in that dominantly fine-grained lacustrine section represents the last large bundle of climatically-forced cyclic lake bed sequences that characterize the lower Portland Formation. Lithologies above these lake beds change to coarse fluvial sandstone packages. The sandstones are the lower part of a much larger fining upwards sequence that becomes redder towards the top. Interdistributary (local) shallow-water red and gray lake beds replace the basin-wide, deep-water gray to black lake beds in the lower Portland Fm. It is within this stratigraphic and paleoenvironmental setting that the K-F quarry plant locality is located. The plant bed is estimated to be ________ meters above the Hampden basalt. No fossil plant localities have been found in the sandstone and conglomerate sequences that make up most of the remaining __________ meters of upper Portland Formation.
The palynoflorules from the Agawam bridge locality are the most diverse from the Portland Formation, containing 17 spore and 20 pollen taxa (Table II) compared to five spore and 15 pollen taxa at the K-F quarry locality. Densoisporites sp., Cycadopites cf. C. jansonii Pocock, and C. durhamensis Cornet & Traverse are the only taxa present at the K-F quarry locality which are not present at the Agawam bridge locality. The lower percentage and diversity of spore taxa and higher percentage of cycadophyte pollen at the K-F quarry locality are consistent with a drier floodplain habitat (see Locality and Stratigraphy section above). The presence of Converrucosisporites cameronii, which Cornet and Traverse (1975) recovered from sporangia of Clathropteris meniscoides, is also consistent with a drier floodplain paleoenvironment containing shallow ephemeral lakes. This dipteridaceous fern was found preserved in situ in both fluvial sandstone and red mudstone environments of the Newark and Hartford basins (Cornet, 1977).
The Sinemurian-Pliensbachian age of the K-F quarry locality is based on four independent pieces of evidence: Diagnostic late Liassic palynomorphs, late Sinemurian beetle elytra, rapid sedimentation based on Milankovich cycles, and a paleoequatorial position during deposition.
Table I
Morphotype or Taxon
Percentage in gray shale
Percentage in red shale
pollen
95.9
97.2
spores
04.1
02.8
Corollina total
88.1
95.9
C. torosa
31.6
40.3
C. torosa non-striate
27.1
34.8
C. torosa striate
04.5
05.5
C. meyeriana
45.7
51.5
C. murphyi
04.5
02.4
C. simplex
01.3
00.7
Corollina endexinal bodies
05.0
01.0
Exesipollenites tumulus?
01.0
00.0
Bisaccate pollen
00.5
00.0
(Alisporites thomasii and Pityosporites parvisaccites)
Araucarian pollen
03.2
00.4
(Araucariacites australis, A. fissus, and A. punctatus)
Cycadophyte pollen
03.2
00.4
(Cycadopites deterius, C. durhamensis, C. jansonii, and Granamonocolpites sp.)
Other pollen
00.0
00.5
(Camerosporites reductiverrucatus)
Articulate spores
02.7
00.7
(Pilasporites allenii)
Pteridophyte spores
01.4
02.1
(Converrucosisporites cameronii, Densoisporites sp., Perinosporites cf. pseudoreticulatus, Todisporites rotundiformis)
|
Table II |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The presence of diagnostic late Liassic taxa such as Callialasporites trilobatus and Staplinisporites caminus (Cornet, 1977), as well as taxa that closely resemble Callialasporites dampieri and Leptolepidites major, favors a late Liassic or younger age (Srivastava, 1987). However, cyclostratigraphy for the lower Portland Formation recently demonstrated the possibility that the lower part of that formation spans as little as 2 million years, making even a Sinemurian age a potential stretch of the data (Olsen, pers. comm. 1993; Cornet, 1993). Because some of these taxa (e.g. Callialasporites dampieri) have been recorded near the paleoequator in Africa during the Hettangian (Reyre, 1973), their presence in the lower Portland Formation of North America has to be balanced against other evidence. Since the Hartford basin was located near the paleoequator during most of its depositional history (Witte et al., 1991), a northward migration of these taxa in the late Liassic from near their place of origin cannot be discounted.
Also present at the K-F quarry locality are insect fossils. They occur mostly in the interdistributary red claystone lake beds. Beetle elytra are most common, but cockroach wings and Orthoptera legs (crickets; grasshoppers) have also been found. One type of beetle elytron stands out as very distinctive because of an elliptically banded dark and light ridge/groove pattern (see below). Elytra of this type are identical to those of Holcoptera giebeli Handlirsch, illustrated by Whalley (1985) from the late Sinemurian of Dorset, England. Taken together with the palynoflora, a late Sinemurian-early Pliensbachian age is given for the K-F quarry locality as a compromise between so many palynomorph taxa common to the late Liassic in older strata and a rapid sedimentation rate indicated by cyclostratigraphy. Additional structural, lithostratigraphic, and magnetostratigraphic evidence is needed before a final verdict on age can be given.
cockroach wing
Materials and Methods
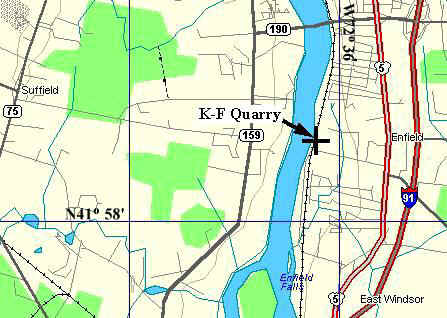
All of the specimens described in this paper come from strata of the Hartford, Deerfield, and Newark basins. The two new taxa and associated palynoflorules come from the same beds in the former K-F (Kelsey-Ferguson) quarry, which is no longer accessible. The quarry was abandoned and reclaimed (filled in) in early 1992 soon after the collection was made by the junior author. The quarry is located near Suffield, CT, in the Broad Brook 7 .5 minute quadrangle at 41 degrees 58' 45" North; 72 degrees 36' 30" West.
The collection of plant specimens is very large, and consists of about 200 pieces of rock, some containing numerous vegetative and reproductive axes. This collection was originally kept at the Westminster School in Simsbury, CT, and was curated by N.G. McDonald. The collection used is this study is now on loan to Dr. Brian Axsmith, Professor of Paleobotany at the University of Southern Alabama (baxsmith@jaguar1.usouthal.edu). Axsmith is planning on writing and publishing a joint paper, which will replace this one. The type specimens and all other specimens used in this study are deposited in the paleobotanical collection at that university. Accession numbers for those specimens have not yet been provided. In addition to plants, various types of insect fossils were found, along with ostracods, conchostracans, and fish. Lithologies in which the specimens are preserved range from red claystones and siltstones to gray claystones and siltstones.
Very little work was required to adequately expose specimens for study and photography. Some degaging was done (degaging is the method of uncovering a specimen using needles to remove sediment). Cuticles and pollen from cones were prepared by standard palynological techniques using HCl, HF, and Schultze's solution for oxidation. Some specimens of cones preserved in gray siltstone were treated with dilute HCl to remove surface coatings of calcite and gypsum, as well as other soluble minerals that obscured detail. One of those specimens was further treated briefly with HF to lighten the gray background and improve contrast for photography. That specimen was then coated with polyurethane to prevent surface damage from handling. Palynological samples from the red and gray claystones were prepared by standard techniques, and the processed residues mounted on coverslips using polyvinyl alcohol. The converslips were then mounted on standard microscope slides with clear bioplastic resin. Photographs of macrofossils were made with a Minolta 35 mm reflex camera using T-Max 100 ISO B&W film, 58 mm lens (1:1.4), compact bellows and/or close-up lenses. Photographs of microfossils were made using a Nikon FX-35A camera attached to a Nikon Optiphot microscope with an A60 dry lens. All photographs were printed on AGFA multicontrast V paper.
Preservation
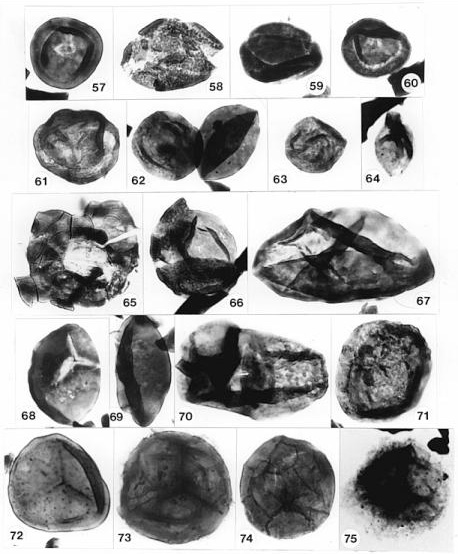
Fossil preservation ranges from very good to poor. Cuticle preservation is marginally good, because weathering has caused fragmentation to such an extent that only small pieces of leaf cuticle can be recovered (Figs. 54 and 65). The condition of those pieces is usually good to excellent. Palynomorph preservation ranges from poor due to corrosion and loss of finer exine details, to very good with internal wall structure at least partially preserved (e.g. Figs. 57- 75). But the most interesting preservation occurs in the brick red claystones, which represent interdistributary shallow lake deposits on an active floodplain. Palynomorph preservation in the red claystones is better than in the gray claystones, which contain more abundant organic material and more evidence of corrosion.
Thermal maturity of kerogen also reflects a difference, with TAI = 3 to 3+ in gray claystone and TAI = 3+ in red claystone. The slightly higher maturity in the redbeds may be due to some oxidation of the organic material prior to diagenesis. But that slight difference does not explain how the palynomorphs could escape complete oxidation in such a hostile environment for preservation. Redbeds are almost invariably devoid of organic matter, with the only other exception noted below black lacustrine limestone layers of the Washington Valley Member in the Feltville Formation of the Newark basin (Cornet, 1977). There in a bright red claystone containing varicolored root traces, palynomorphs are moderately well preserved. But at the K-F quarry no such reducing environment exists above the redbeds that could explain a rapid switch from oxidizing to reducing conditions as occurred when the Washington Valley lake rapidly transgressed the basin. The only explanation that makes geologic sense is rapid burial in an active floodplain. The copper minerals may have played a role in early diagenesis if copper salts were present in the ground water system. Copper may have sterilized the sediments from any further bacterial-fungal decay, and changed the EH potential of the sediments. The presence of malachite and calcosite haloes around most plant megafossils in the red claystones implies an early diagenetic interaction, as do concretions, which are also present and which enclose some plant megafossils (e.g. Fig. 29).
Systematics
Family: Cheirolepidiaceae
The diagnosis for this family, emended by Clement-Westerhof and Van Koniknenburg-Van Cittert (1991), is modified here:
Diagnosis: Ovuliferous cones compound; scales of the ovuliferous dwarf-shoot range from considerably to completely connate. 1-2 ovules per dwarf-shoot, invertedly oriented (micropyle proximally directed) or uninvertedly oriented (micropyle distally directed) on the adaxial side of the dwarf-shoot; ovules/seeds covered by an epimatium with each enclosed by separate cuticles.
Subfamily: Cheirolepidoideae subfam. Nov.
Diagnosis: Dwarf-shoot with no specialized lobes on scale; ovule(s) inverted (micropyle proximally directed) or not inverted.
Hirmeriella estonensis (Kendall) Cornet and McDonald nov. comb.
Basionym: Araucarites estonensis Kendall 1952.
Remarks: H. estonensis is associated with P. araucarinum (Pomel) Saporta (= P. connivens Kendall 1952: Van Konijnenburg-Van Cittert, 1987). Kendall (1952) provisionally placed this taxon in the Araucariaceae. Male cones associated with P. araucarinum contain Corollina torosa pollen (Couper, 1958; Van Konijnenburg-Van Cittert, 1971). The resemblance of H. estonensis to cheirolepid dwarf-shoots of the Newark Supergroup supports palynologic evidence that this taxon belongs to the Cheirolepidiaceae and not the Araucariaceae, despite the leaf-taxon name having priority: P. araucarinum. Van Konijnenburg-Van Cittert (1987) made an illegitimate transfer of A. estonensis to Hirmeriella, but in a later paper did not recognize that transfer (Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991).
Subfamily: Conanthoideae subfam. nov.
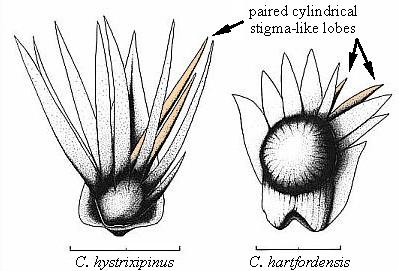
Diagnosis: Dwarf-shoot with two, paired central lobes modified, cylindrical to oval in cross section rather than dorso-ventrally flattened, and significantly different in length; ovule(s) not inverted (micropyle distally directed).
Remarks: Hirmeriella does not possess these hollow specialized lobes containing extensions of the ovary cavity and its cuticle into their bases.
Conanthus Cornet and McDonald, gen. nov. (natural genus).
Derivatio nominis: Conanthus - Con, after conus, Latin for cone; anthus, after anthos, Greek for flower; Conanthus, meaning a cone that resembles a flower, particularly during its early stages of development, and a flower that becomes a cone at later stages of development.
Diagnosis: Dwarf-shoots possessing ten distal lobes arranged in two rows; adaxial row with six scales, abaxial row with four scales; two adjacent lateral lobes in abaxial row hollow and cylinderical or oval, i.e. lens-shaped in cross section instead of solid and flat; space within these lobes connected with superior part of ovary chamber; outermost lobes on scale much smaller than adjacent more interior lobes; proximal suture on ovary developed asymmetrically more on one side (longer) than the other.
Conanthus hystrixipinus Cornet and McDonald, sp. nov. (natural species).
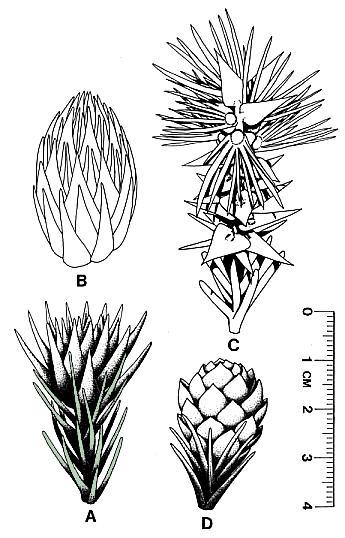
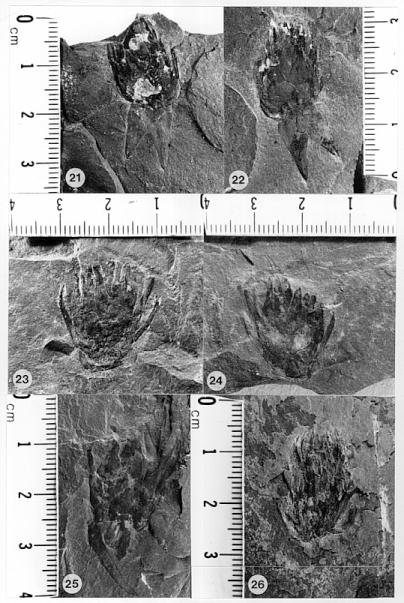
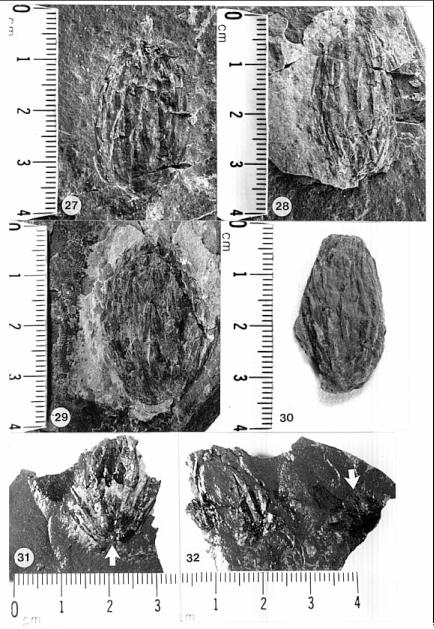
Figures: 1-3, 9, 13-14, 15-20, 21-26, 27-32, 33-34, 46-56.
Paratypes: (15,16,17-18,20,21-22,23-24,25,26,27,28,29,30,31-32).
Depository of specimens:
Type locality: Abandoned and reclaimed K-F quarry, Suffield, Connecticut, USA.
Stratigraphic position: lower middle Portland Formation.
Age: late Sinemurian-early Pliensbachian, Early Jurassic.
Derivatio nominis: hystrixipinus - Hystrix, a genus of porcupine, in reference to the resemblance of mature cones at the time of seed dispersal to a porcupine with its quills extended; Pinus, for the pine-like aggregates of elongate lobes on each bract-scale complex, aggregates which resemble fascicles of pine needle.
Diagnosis: Dwarf-shoots possessing ten distal lobes arranged in two rows; adaxial row with six lobes, abaxial row with four lobes; lobes 9-17 mm long, 1-2 mm wide at base, tapering gradually to a point; two adjacent lobes in adaxial row hollow and cylinderical or oval; these lobes 1 mm wide at base but distinctly different in length (11 mm and 16 mm); space within these lobes connected with superior part of ovary chamber; central cylinderical lobes oriented or directed laterally; outermost lobes on scale much smaller than adjacent more interior lobes; scale and lobes asymmetrically distributed, as is suture on ovary wall; suture developed more on one side (longer) than the other; one seed per dwarf-shoot; mature seed oval to rectanguloid in shape, 5.0-6.0 mm x 4.0-5.0 mm; scale cuticle papillate like that of leaf; scar for attachment to bract located at middle of body of scale in center of seed compression.
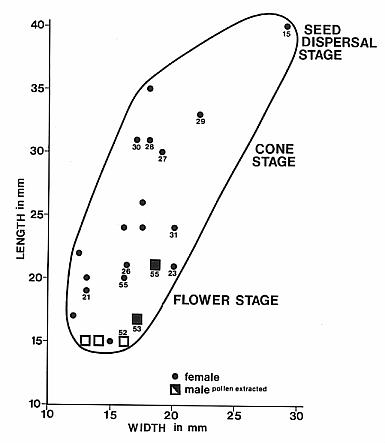
Female cones attached on short side branches 1.5 cm to 2.0 cm long; female cones heteromorphic, changing through ontogeny: early stage flower-like with bracts developing before ovuliverous scales, 2.0 cm in length, bracts crowded into tight spiral with tips terminating at about same level above base; bracts sagitate with wide bases and long tapering pointed apices; dwarf-shoots develop later, not obvious in early flower stage; cone axis elongates to 3.5 cm following flower stage, and dwarf-shoots develop elongate lobes that project distally as far as tips of bracts; submature cone ovoid in shape; mature cone with reflexed outward-oriented bracts that expose dwarf-shoots; bract cuticle papillate like that of leaf.
Male cones attached on short side branches, about 1.2 cm long; male cones approximately spherical, 15-20 mm in diameter; microsporophylls spirally arranged, peltate in attachement, rhomboidal in shape with acute apex; pollen sacs attached near juction of sporophyll stalk and head (number and shape of sacs undeterminable); male cones more rosette in shape at early stage of development; cone axis elongates with increase in size at maturity; cuticle papillate like that of leaf.
Pollen of the Corollina torosa type, spheroidal with flattened poles, 22-29 mu in diameter; ectexine more glassy and transparent than endexine, intrabaculate with thickened equatorial band.
Foliage of the Geinitzia/Elatides type, helically arranged with free part falcate or spreading; shape variable depending on growth stage and branch order: Dorso-ventrally flattened with prominent midvein or keel, apex rounded to acute; leaf sometimes needle-like in form (i.e. equal thickness in horizontal and vertical dimensions); proximal portion of leaf merges into a basal cushion without contracting; leaves typically (but not consistently) variable in length along axes, gradationally alternating in cycles from short leaves (0.5 cm to 0.6 cm in length) to long leaves (1.4 cm to 2.2 cm in length), and then back to short leaves at intervals ranging from 3.0 cm to 6.5 cm; leaf width at base varies from 1.8 mm to 2.2 mm, proportionate to length.
Leaf cuticle papillate; ordinary epidermal cells rectanguloid, and arranged/oriented in longitudinal files; epidermal cells more isodiametric in shape around stomata, forming two rings of subsidiary cells around each stoma; outer ring consists of about six more or less isodiametric cells, while inner ring consists of about five wedge-shaped cells; all epidermal cells possess one papilla; outside stomatal rings papillae are more or less centrally positioned within margins of cells; papillae of outer ring also centrally positioned, but papillae of inner ring skewed towards guard cells and occasionally fused laterally to form elongate ridges bordering stoma; guard cells sunken below inner ring of subsidiary cells; stomatal opening about 25-26 mu in diameter; inner subsidiary ring about 38-45 mu in diameter; outer subsidiary ring about 122-162 mu in width.
Branching with minimum of four orders: First order axis 4.0 mm wide at base; third order axis 3.0 mm wide at base; second and third order branching paradichotomous to parapinnate; fourth order branching parapinnate (sometimes opposite); second order branches 11.5 cm to 18.0 cm long, third order branches 1.0 to 11.0 cm long, fourth order branches 1.0 to 2.5 cm long; average internode length on second order branches varies from 2.5 cm to 4.5 cm; average internode length on third order branches varies from of 2.9 cm to 5.0 cm, with internode length generally decreasing in value towards branch apex (maximum 10.0 cm; minimum 1.0 cm).
Trunks (in same layers as branches and cones) small, permineralized, partly compressed, 6.0 cm to 13.0 cm in width.
Description with interpretation: The abundance and diversity of organs belonging to one species, Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov., allow us to reconstruct this Jurassic conifer nearly completely. Most of its organs are in organic attachment, with the only exception being the larger branches and trunks found in this assemblage. C. hystrixipinus was a tree of small to moderately size with profuse branching (Fig. 2). It possessed some of the longest leaves (2.2 cm) yet recorded for any member of this family (Figs 1-3, 9, 13-14). The leaves vary in size and form along various shoot segments from small leaves (5-6 mm long) that approach Elatides Heer (leaf/cone form-genus) in size (compare Figs. 8 and 9) to very long leaves (Figs. 1-3) that resemble those of Cunninghamia (extant Taxodiaceae: Miller, 1990). The cyclic variation in leaf length may have been a response to a seasonal climate, implying that there was a period of the year when growth conditions were optimum, and a period when they were not. If this is the case, then the transition from unfavorable to favorable growing conditions was more gradual than the transition from favorable to unfavorable, because change in leaf length is more abrupt from long to short, and new side branches and shoots begin with short leaves (Fig. 3, 9), implying that branch "buds" were produced at the end of a favorable growing period.
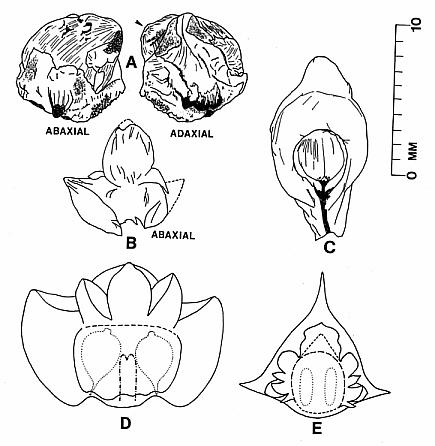
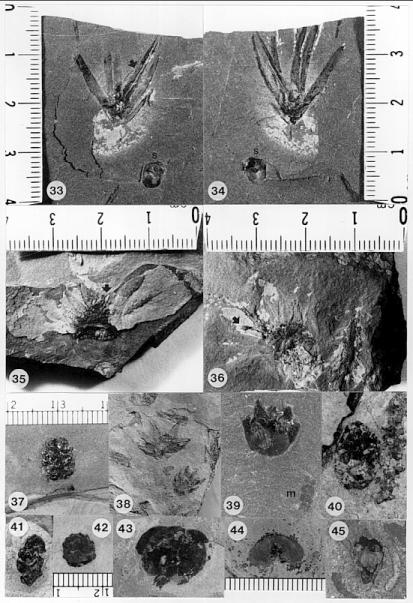
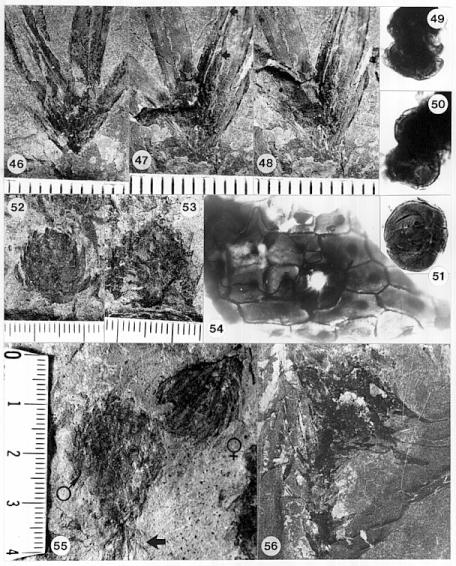
The cones of C. hystrixipinus differ from those of most conifers, as well as other Cheirolepidiaceae, in their transformation from flower-like reproductive structures when they first unfold (Figs. 21- 24, 55) to conventional strobili near maturity (Figs. 27-30). At an early stage the cones appear as a tight rosette of elongate bracts with no lobes of dwarf-shoots visible (Text-figs. 3A-3B). In between the early and late stages the cone axis elongated as did the lobes on the dwarf-shoots (Figs. 25-26, 31-32; Text-figs. 3D-3E). The tentative number of scale lobes in this subfamily (based on two Conanthus species) is ten, arranged in two rows (six in the adaxial row; four in the abaxial row). In the mature dispersed dwarf-shoot (Figs. 19, 33-34) two of these lobes stand out as darker and more carbonaceous; they are located next to one another in the abaxial row next to two large flat lobes (Text-fig. 5). The round and flat lobes in the abaxial row appear to have developed early, followed by the elongation of the remaining lobes in the adaxial row. Preservation is not good enough to allow isolation of individual scales via maceration of cone compressions: Cuticles are highly fragmented by weathering.
Figures 31 and 32 show a cone at an intermediate stage of development which broke off slightly above its base. On the back side of the specimen two adjacent and basally connected lobes of one dwarf-shoot can be distinguished (Text-fig. 3E'). These appear to be the specialized round lobes whose hollow centers communicate with the chamber or pouch containing the ovule (Figs. 33-34, 46-48; Text-fig. 3.5). Their hollow condition is based on specimens where these lobes are filled as far up as 50% of their length with the same type of red granular material that fills the ovary chamber (Fig. 47, arrows). No similar type of material can be seen in the flat lobes. Similar red granular material fills the hollow lobes of dwarf-shoots of another Conanthus sp. (Figs. 35-36, arrows). Even though they appear hollow, there is more carbonaceous material associated with them; the distribution of this organic material along a narrow band (Fig. 47) implies the presence of vascular material. It would seem logical that if they had a specialized function in pollination (i.e. functioned as stigmas), they would be more heavily vascularized than the other lobes.
The paired central lobes appear to have developed first, partly because of the larger size of those lobes (they had an earlier start in development), but mainly because they appear early in cones of intermediate development (Text-figs. 3F, 3G, 4B). Had all the flat lobes on the scale developed at the same time, compressions with 32-38 scale complexes would appear as a mass of overlapping structures of which individual scales and bracts could not be readily distinguished (contrary to Text-figure 3). The fact that the bracts developed much earlier than the dwarf-shoots also implicates tiered levels of differentiation. Recognizing these lobes on cones of intermediate development and recognizing their specialized function are important for interpreting the reproductive strategy and apomorphies of this plant.
scale in millimeters
Associated numbers correspond to photographs in plates.
By the seed-dispersal stage of development the cone axis had elongated about 3.5 times that at the flower-like stage (Text-fig. 6; compare Figs. 15 and 23-24; Text-figs. 3A-3B and 4C). Mature cones attached to their leafy shoots have been found (Figs. 13, 15), as well as distal portions of mature cones that apparently broke off above the base of the cone (Figs. 17-18). Interpreting these as incomplete or as fragments is based largely on the attachment of proximal portions of mature cones on some branches (Figs. 13-14, 16, 56). In these examples numerous lobes of mature bract/scale complexes can be distinguished, even though the number of such complexes and the size of the cones are significantly less than that of a complete cone (cf. Text-fig. 3). The incomplete nature of some dispersed cones and the number of cone bases still attached to their branch axes (e.g. Fig. 14, arrow) indicate that the cone axis was fragile and susceptible to breaking. That condition can be best explained if the cone axis was poorly lignified and/or contained a large central pith. Direct evidence that it was weak comes from specimens where the axis can be observed. In Figure 31 (arrow) the axis appears as a ring of carbonaceous material filled with sediment (Text-fig. 3E). Indirect evidence that it was weak comes from the amount of cone elongation following the flower-like stage (Text-fig. 6). In no specimen of female cone does the axis stand out as thickly carbonized.
At the time of seed dispersal, the bracts reflexed/recurved backwards to expose the digitate seed scales. Attachment position on the bract can be only approximated by the position of the scales on the bracts, since that connection is small, based on the scar visible on the dwarf-shoot (Fig. 48, arrow). The appearance of a mature cone with dozens of ten-lobed seed scales projecting outwards is reminiscent of a porcupine (Figs. 15, 17-18), an appearance that we reflect in its name, C. hystrixipinus (Text-fig. 4C). No other member of the Cheirolepidiaceae has been described with scale lobes even half as long as those of this species. Their function undoubtedly served in seed dispersal, since the lobes probably projected away from one another at maturity (aided by the reflexing of the bracts to create enough space for the lobes to separate after drying), creating a helicopter type of diaspora (Text-fig. 5).
Only a few isolated scales were found that show the seed still in the pouch or ovary (Fig. 17-18, 20; Text-fig. 3.4). It is oval to rectanguloid in shape, 5.0-6.0 mm x 4.0-5.0 mm in size. Based on its size, there is only room for one seed per dwarf-shoot. A possible dispersed seed (one that separated from its scale) is preserved just below the most complete and best preserved dwarf-shoot of C. hystrixipinus (Figs. 33-34, s). Since 1) there is no developed seed present inside the ovary of that scale (the ovary is partly collapsed and filled with organic and inorganic residue), 2) a seed is present just below that scale, and 3) that seed resembles in size and shape outlines of seeds present in scales in Figure 20, it is tempting to think that this associated seed came from that scale (Text-fig. 3.5). The association, however, may be mere coincidence; there is nothing other than size and shape to indicate that the seed belongs to C. hystrixipinus.
The ovary is filled with a combination of silica, gypsum, and calcite cements that bind together irregular organic masses with a bright red granular material (Figs. 33-34). The red material is abundant, transparent, and amorphous, is not soluble in organic solvents or strong inorganic acids (e.g. aqua regia), and is brittle, implying that it is a high molecular weight resin-like material. This red material is distributed throughout the ovary chamber of several dispersed scales (Figs. 33-36), sometimes surrounding the ovule. It extends up into the hollow portions of the paired stigma-like lobes as much as 50% of their length (Fig. 47, arrows). The distribution of this material demonstrates a communication between the ovary chamber and the stigma-like lobes, and implies the existence of an organic fluid or substance that may have been derived from micropylar "pollen drop" exudate. Similar resin-like material has been described inside the ovary of Hirmeriella muensteri and H. kendalliae (Text-fig. 7; Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991; Harris, 1979).
The fact that some dwarf-shoots can lose their seeds during transportation is important in the recognition and interpretation of features of the ovary wall. Here the ovary is defined as the pouch, comprised of wall and enclosed space containing the ovule/mature seed, which was formed by the scale proper on the abaxial side, and by an epimatium or flap of the scale that completely covered the seed on the adaxial side (Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991). The paired hollow stigma-like lobes at the apex of the ovary are also considered to be part of the ovary, because the spaces within them communicate with the ovary chamber (see description above). The seed can apparently fall out of the ovary during transportation. The mechanism appears to be via a suture that runs across (tangential to the cone axis) the base of the ovary sac or scale, below the point of vascular attachment of the scale to the bract. The thin flap-like nature of that opening or suture can be distinguished in the specimen that lost its seed (Figs. 46-48). The suture appears to run from near the base of the lobe on one side to near the base of the lobe on the other (Text-fig. 5), and is asymmetrically distributed because the lobes are asymmetrically positioned around the ovary. The suture is long enough to allow a fully developed seed to exit the ovary once its vascular attachment to the scale is severed. The orientation of the ovule within the ovary will be discussed in the next section, where additional evidence from other cheirolepidaceous scales will be presented.
One of three male cones, which contains Corollina pollen (not as well preserved as that from the cone in Fig. 53), was found attached to a poorly preserved short leafy shoot (Fig. 55, arrow), the leaves of which are bent backwards slightly. Because it is attached, it is included in the species, Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov., rather than placed in a new species of the genus, Classostrobus. The male cones at pollen maturity (based on extracted pollen) somewhat resemble the flower-like stage of female cones in size and overall shape (compare Text-figs. 4A and 4D). This resemblance is based on a specimen of a male cone found lying next to an immature female cone, which is distinguished by its much longer bracts (Fig. 55). Other mature male cones are present in that layer (Figs. 52-53), implying that they were transported and deposited together at the time of pollination. Both male and female cones at this stage have short cone axes, and their bracts are tightly arranged in a rosette pattern. The male cones are very large compared to pollen cones of other cheirolepidaceous taxa from older Jurassic beds (Figs. 37, 40-42). They are at least 70% larger than the average size of all other Classostrobus cones (those producing Corollina pollen) found in the Newark Supergroup.
The pollen extracted from one of the three male cones (Fig. 53) is well enough preserved to be identified to species. It is Corollina torosa (Figs. 49-51). It possesses the distinctive intrabaculate ectexine of that taxon. C. torosa makes up 31.6% of the gray shales at the locality, and 40.3% of the red shales (see section above on preservation). Corollina spp. make up 88.1% of the gray shales and 95.9% of the red shales, with a ratio for C. torosa to C. meyeriana of 0.69 for the gray shales and 0.78 for the red shales. C. torosa is distinguished from C. meyeriana by an intrabaculate ectexine, which is striate (intrabaculi aligned) in about 14% of the specimens. Poor preservation of some specimens reduces the structure of the ectexine, making it impossible to distinguish between the two species, and raising the possibility that C. torosa may be more abundant than can be determined from the state of preservation. Other palynoflorules from the Portland Formation (Cornet, 1977), where preservation is good, contain high percentages of the massive-walled C. meyeriana, indicating that the ratios given above are probably close to being correct. It is important, however, to recognize that Conanthus hystrixipinus produces the type of Corollina that has been implicated in the development of incompatibility systems within this family (Taylor and Alvin, 1984).
Conanthopsis Cornet and McDonald, gen. nov. (form-genus).
Derivatio nominis: Conanthus with the substitute ending opsis, indicating a form-genus status for dispersed dwarf-shoots conforming to the diagnosis of same organs of Conanthus (sensu Poort and Kerp, 1990).
Diagnosis: Dwarf-shoots possessing ten distal lobes arranged in two rows; two adjacent lobes in abaxial row hollow and cylinderical or oval instead of solid and flat or lens-shaped in cross section; space within these lobes connected with superior part of ovary chamber; outermost lobes on scale much smaller than adjacent more interior lobes; suture developed asymmetrically more on one side (longer) than the other.
Conanthopsis hartfordensis Cornet and McDonald, sp. nov.
Holotype: (35-36).
Paratype: (32).
Depository of specimens:
Type locality: Abandoned and reclaimed K-F quarry, Suffield, Connecticut, USA.
Stratigraphic position: lower middle Portland Formation.
Age: late Sinemurian-early Pliensbachian, Early Jurassic.
Derivatio nominis: hartfordensis, after Hartford basin, Connecticut, where this taxon was found.
Diagnosis: Dwarf-shoots possessing ten distal lobes arranged in two rows; two adjacent lobes in abaxial row hollow and cylinderical or oval instead of solid and flat or lens-shaped in cross section, 4 to 7 mm long, 1.0-1.5 mm wide at base; space within these lobes connected with superior part of ovary chamber; outermost lobes on scale with short free tips, 1 cm long, extending as 2.5 mm wide flanges along the sides of the ovary down to its base, and attached along one side; body of scale (excluding distal lobes) 12 mm long by 9-10 mm wide; central flat lobes 7-8 mm long, 2.0-2.5 mm wide at base; suture developed across the base of scale; one seed per dwarf-shoot; seed compression about 8 mm in diameter; scar for attachment of scale to bract located at middle of body of scale in center of seed compression
Description with interpretation: This taxon is based on three dispersed dwarf-shoots (Figs. 32, 35-36). The general morphology and number of distal lobes on this scale is identical to that of Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov. Moreover, the paired stigma-like lobes, even though shorter, are distinctly unequal in length like those of Conanthus, and are much more carbonaceous than the flat lobes on either side, which are preserved only as faint impressions. The hollow nature of the stigma-like lobes was determined by the red granular material which fills their bases up to 30% of their length, and which is continuous with similar red material that fills the ovary space just above the ovule. Here we have preserved in the same deposit two distinct dwarf-shoot taxa that demonstrate the unique and distinguishing features of the subfamily, Conanthoideae.
Large enough cuticle fragments from these specimens could not be recovered, but based on observation under a high powered binocular scope, the cuticle is papillate like that of Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov.
Careful removal of cements with dilute acid from the stigma-like lobes on the holotype (Fig. 36) exposed the delicate (fragmented) cuticles. Removal of organic pieces from the lobes through imbibing with a glycerol/water mixture using a micropipette obtained recognizable fragments of cuticle, as well as several specimens of Corollina. One specimen (C. meyeriana) was light and transparent like that of dispersed pollen in the matrix, indicating that it was probably derived from there. The other two specimens were nearly opaque and colored (unidentifiable to species) with the same type of dark organic material that coats the cuticle and stains adjacent pieces of cement. We suspect that these specimens of Corollina were attached to the outside surfaces of the stigma-like lobes (one specimen was attached to a cuticle fragment). The presence of pollen on the lobes proves nothing other than that pollen landed there. But it does demonstrate that the lobes could have functioned as stigmas, allowing pollen tubes to penetrate the cuticle and grow down their hollow centers to the ovule micropyle (analogous to the condition in angiosperms). In angiosperms we know the function of the stigma and style. Therefore, comparable morphology in a Cretaceous angiosperm implies comparable function (e.g. Drinnan et al., 1991). But we do not have a living example of a conifer with a stigma/style, and at best we can only implicate function for the stigma-like lobes.
The only other leaf type preserved at the K-F quarry locality is illustrated in Figure 7. It falls into the form-genus, Pagiophyllum, and does not appear significantly different in form from the more common types of leafy shoot found at other Early Jurassic plant localities of the lower Portland Formation and upper Turners Falls Formation, Deerfield basin (e.g. Figs. 4-6, 8, 10). If Conanthopsis hartfordensis Cornet and McDonald gen. et sp. nov. belongs to this leaf type (Fig. 7), the shorter lobes on the scale are consistent with shorter leaves, just as the extreme length of lobes on the scales of Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov. match the extreme length of leaves for a cheirolepidaceous conifer. Because no cuticle was recovered from this specimen, it is not described as a new species. A papillate cuticle, however, can be detected under binocular magnification. Such a cuticle distinguishes this taxon from similarly shaped leaves of the lower Portland Formation (e.g. Fig. 4), which have a smooth non-papillate cuticle (Text-fig. 8: Floral Assemblage D).
For clarity and distinction in floral comparison below, the older leaf type (Figs. 4, 5, and 10; Text-fig. 8: P13s) is provisionally placed in one of Newberry's (1888) taxa from the Hartford basin, which is distinguished only by leaf shape and size:
Pagiophyllum simile (Newberry 1888) Cornet and McDonald comb. nov.
Basionym: Pachyphyllum simile Newberry 1888, p. 88, Pl. XXII, Fig. 2.
Evolution and climatic trends in the Newark Supergroup
Cheirolepid foral assemblages A through E
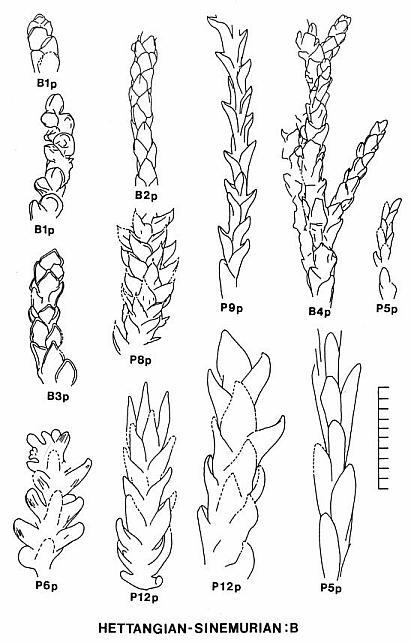
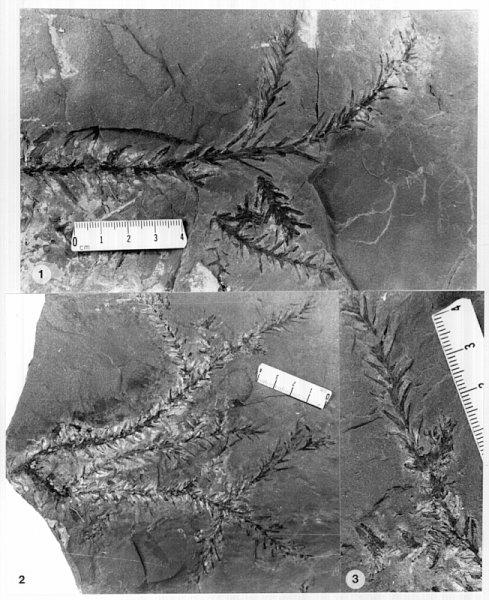
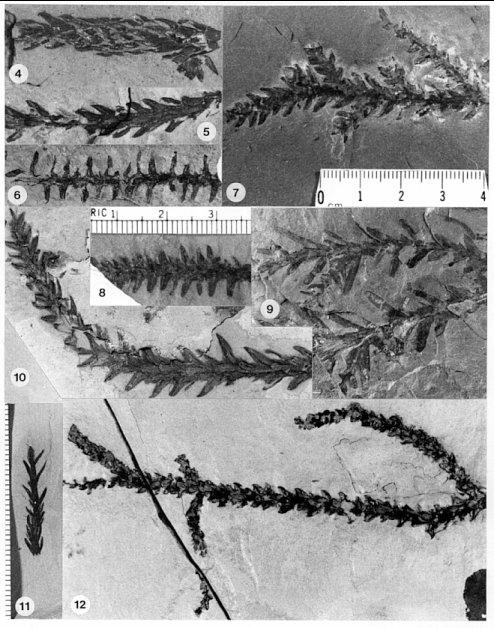
The basic branch habit and leaf morphology in the Cheirolepidiaceae is very similar to that of many Paleozoic conifers, with the only significant distinction being a less organized and more random distribution of ultimate branches (e.g. Figs. 2, 7, and 12). In addition, the basic dwarf-shoot of Hirmeriella muensteri resembles that of the Majonicaceae (Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991). Leaves for the earliest Jurassic members of this family (Floral Assemblage B: Text-figs. 8-9) are typically small and bract-like: Hence the name Brachyphyllum. Some of these taxa have more elongate leaves (e.g. Fig. 11), but overall leaf size for ultimate shoots is small (5 mm or less in length). This distinction is blurred for larger and older branch axes, where leaves can be much larger (up to 8 mm in length). A similar range of leaf sizes for Brachyphyllum was found in Rhaetian-age Triassic strata containing abundant Corollina pollen (Floral Assemblage A: Text-figs. 8-9; Cornet, 1977; Cornet, 1989).
At the end of the Triassic a major period of floral extinction is recorded in the Newark Supergroup and elsewhere in North America (Olsen et al., 1989; Cornet, 1989; Olsen et al., 1990; Cornet, 1993). Of the 42 palynomorph taxa identified in the latest Triassic sediments of eastern North America (Cornet, 1993), only 10 survive the boundary event and continue into the Jurassic. Numerous other unidentified (unnamed) taxa also became extinct, bringing total palynomorph extinction to more than 80% (Cornet and Olsen, 1990). Similar extinctions and turnovers, including vertebrates and invertebrates, have been recorded almost everywhere in Pangaea at the end of the Triassic (e.g. Cornet and Olsen, 1985; Olsen et al., 1989; Olsen et al., 1990). It is at this time of global change (basal Jurassic) that the Cheirolepidiaceae came to dominance, even though they competed successfully against other conifers in the latest Triassic, with their pollen increasing to palynofloral dominance at various times in the Rhaetian (e.g. Venkatachala and Goçzan, 1964; Morbey, 1975; Cornet and Olsen, 1985).
All seven leaf types recorded in the earliest Jurassic of the Newark Supergroup (Cornet, 1989: Feltville, Shuttle Meadow, and Midland formations) possess a similar type of papillate cuticle and stomatal apparatus (Cornet and Traverse, 1975: Pl. 6, figs. 1-2). This type of cuticle is identicle to that of associated dwarf-shoots (Text-fig. 7), and to that of Brachyphyllum scottii Kendall 1949 (form-genus) from the earliest Jurassic Larachbeg marine beds, Morvern, Scotland. Leafy shoots and branch systems of B. scottii (Fig. 12) are common in the oldest Jurassic beds of the Newark Supergroup, along with dispersed dwarf-shoots (Text-figs. 8-9; Cornet, 1989). In the Midland and Feltville formations associated dispersed dwarf-shoots contain one enclosed seed and bear either three lobes (Text-fig. 7A: Midland Fm.) or only one distal lobe (Text-fig. 7C: Feltville Fm.). The ovules in these dwarf-shoots were not inverted (Text-figs. 7A and 7C). The female dwarf-shoot described by Kendall (1949) appears to be similar in that it has either very reduced lobes or one very broad lobe (derived from the lateral fusion of lobes?). It possessed a rim that surrounds the multiple cutinized membranes of the seed/pouch like a wing, similar to that of the specimen in Fig. 45 (Text-fig. 7C). Pollen occurring in the shales at Larachbeg is almost all of one type: Corollina meyeriana. C. meyeriana also strongly dominates (90+%) the oldest Jurassic strata containing B. scottii in the Newark Supergroup.
A. Midland Fm., Culpeper basin, VA (USA).
B. Turners Falls Sandstone, Deerfield basin, MA (USA).
C. Feltville Fm., Newark basin, NJ (USA).
D. Hirmeriella meunsteri, Jurassic (Germany).
E. Hirmeriella kendalliae, Jurassic (United Kingdom).Isolated bracts found in equivalent-age beds of the Feltville Formation, Newark basin (also associated with B. scottii leafy shoots), outwardly resemble the dwarf-shoots associated with B. scottii in Scotland (Fig. 43). Unusual ovuliferous scales that bear impressions of two ovules/seeds, lack apical lobes, but possess lateral wings are also present in this basal Jurassic flora (Fig. 44). If they belong to the Cheirolepidiaceae, dwarf-shoot diversity for this family in the earliest Jurassic was anything but conservative, and much experimentation divergent from that of Hirmeriella may have been occurring in this family during its initial radiation.
Together the above facts indicate a related group of taxa that evolved from a common ancestor, which survived the Triassic/Jurassic extinctions. All variations of leaf types could be derived from one taxon, and the more simple shapes of associated seed scales contrasts strongly with the six-lobed dwarf-shoots of Hirmeriella muensteri from the early Liassic. The strong dominance of Corollina meyeriana pollen in these sediments supports this thesis. It is therefore possible that the dominance of papillate cuticle in Floral Assemblage B is caused by both climatic adaptation and default taxonomy, influenced by what taxa survived the Triassic/Jurassic-boundary extinction event.
Because the leaf cuticles of Floral Assemblage B are uniformly papillate, while those of Floral assemblages A and C are uniformly non-papillate and smooth (Text-figs. 8-9), adaptation to different climates is strongly suspected. Non-papillate cuticles with sunken stomata dominate brachyphyll and pagiophyll conifers of the marine- influenced German Liassic (Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991) and Yorkshire Jurassic delta (Kendall, 1947, 1948). However, the nature of climatic change is not intuitively obvious from the study of cuticle alone: Spore abundance and diversity is much greater for Floral Assemblage B than it is for either floral assemblages A or C (Cornet, 1977; Cornet, 1989; Cornet, 1993). Papillate cuticle appears to be an adaptation to high insolation but not low humidity, and the function of papilli may be to scatter and deflect light in order to provide some degree of protection during the dry season of a monsoonal climate, while allowing enough light to enter the leaf for rapid growth during the rainy season. Papillate cuticles are rare to absent in floral assemblages C and D, but return in Floral Assemblage E as depositional environments in the Hartford basin changed from dominantly lacustrine to dominantly fluvial (riparian). Fluvial channel sandstones are also common in strata containing Floral Assemblage B.
Reproductive structures and associated pollen
Three isolated male cones from the Midland Formation, Culpeper basin, VA, were dissected and the pollen studied (Cornet, 1977, p. 188-191). Two small globular cones were similar to that illustrated in Figure 42 from the Feltville Formation, Newark basin, NJ (ERT locality). Both yielded large quantities of C. meyeriana pollen. However, the pollen sacs also contained grains with varying degrees of intrastructural development of the exine. One cone produced an assemblage of C. meyeriana with either massive wall structure (75%) or intrapunctate ectexine structure (25%). Uncompressed microsporophylls of this cone (mounted separately for study) each possessed two sets of four short cylindrical pollen sacs; each set was located on opposing sides of the microsporophyll axis. The pollen sacs of each set appeared to be arranged in a row, although the sacs were frequently wrapped around one another to some degree in the fossil state.
The second small cone of similar size and shape produced an assemblage of C. meyeriana (75%), C. murphyi (22%), and C. simplex (3%). The contents of pollen sacs for each microsporophyll do not appear to have had a uniform assemblage: One fragment of the cone, when squashed under a coverslip, produced mostly C. meyeriana, while another fragment of the same cone produced about equal proportions of C. meyeriana and C. murphyi, along with some C. simplex. The third pollen cone was also globular, but larger. It also resembled in shape the cone in Figure 42 from the Feltville Formation. This cone appears to have had a papillate cuticle based on fragments of such cuticle in the pollen preparation. This cone, however, produced a pollen assemblage dominated by non-striate specimens of Corollina torosa (70%), along with some striate and pseudostriate specimens of C. torosa (4%), numerous specimens of C. simplex (25%), and rare C. murphyi (1%). Interestingly, most of the grains possessed endexinal bodies, which sometimes protruded from ruptured specimens. The proportion of non-striate and striate specimens is similar to that which occurs within the Corollina meyeriana palynofloral zone of mostly Hettangian age (Cornet and Olsen, 1985; Cornet, 1993).
It is unlikely that all three of the above pollen cones were produced by the same conifer species. The two smaller pollen cones may have been produced by the same taxon, but slight differences in pollen morphology could also point to derivation from different species. Differences in morphology are unlikely to have been the result of preservation, because it is excellent. There is also the possibility of hybridization, which could account for the range of pollen types in one cone. Considering the conservative morphology of these plants and their potential close phylogenetic relationships if they were derived from a common ancestor at the beginning of the Jurassic, hybridization is a real possibility. Genetic variation within a population during a radiation must also be considered. But C. meyeriana and C. torosa range back to the Norian, and do not always occur together in the same palynoflorule. C. murphyi, however, has its first appearance in the basal Jurassic. Interestingly, C. murphyi is a very rare palynofloral component until the top of the Corollina torosa palynofloral zone (late Sinemurian-early Pliensbachian), even though it was recorded as a common element in one of the pollen cones.
Floral assemblages and biostratigraphy
The next younger Jurassic flora (Floral Assemblage C) occurs near the top of the East Berlin and Towaco formations (Text-fig. 9), which are separated from near basal Jurassic floras of the Hartford and Newark basins by widespread basalt formations (Olsen et al., 1989). The composition of the flora C is distinctly different from the older flora B, and there appear to be no shared cheirolepidaceous taxa (Text-figs. 8 and 9; Cornet, 1989). The transition between the two floras occurs within the East Berlin and Towaco formations, not far below the youngest basalt flows in the zone of igneous extrusives. Replacement appears to be sudden, and is not associated with any overt change in lithology or stratigraphy. Olsen (1993, pers. comm.) thinks that the entire zone of extrusives (interval of alternating basalt and sedimentary units) spans no more than 800,000 years based on magnetic and Milankovich-type cyclostratigraphy.
Seven leafy shoot taxa are recognized in the flora C (Text-fig. 8), all of which possess a similar type of smooth non-papillate cuticle with sunken stomata. No taxa with papillate cuticles have been detected to date. Both floras B and C contain a mixture of Pagiophyllum and Brachyphyllum species, although Pagiophyllum spp. is taxonomically more abundant in the flora B (5 compared to 3 species). Fern spore diversity and abundance is also greater in the older flora, which is consistent with a shift to a higher level of microphylly in the East Berlin and Towaco formations. Cycadophyte pollen is more abundant in the second Jurassic flora, and evaporite crystals are more common in shore facies of basin lakes (Lorenz, 1988). All these facts indicate a shift to more arid conditions within the Hartford basin as a plausible cause for the floral turnover.
Dispersed dwarf-shoots in flora C are also different. They are distinctly 5-6 lobed, with some of them resembling the scales of Hirmeriella muensteri (Fig. 38). Leaves similar to that species are also present, implying that this flora may have immigrated into the area rather than evolved from a taxon of the older flora. H. muensteri and other members of the family have been implicated as coastal halophyte, having a habit of either salt marsh shrub or mangrove-like tree living in an intertidal area (Upchurch and Doyle, 1981; Francis, 1983). Increased frequency of Corollina pollen in nearshore marine sediments has been taken as evidence that the parent plants dominated coastal areas (Pocock and Jansonius, 1961; Wall, 1965; Hughes, 1975, 1976). Cheirolepidaceous conifers related to coastal marine taxa may have bordered the alkaline to brackish water rift basin lakes during this period of time. Interestingly, the palynoflora shows no significant change, with C. meyeriana continuing to dominate until near the middle of the stratigraphic interval containing flora C (Text-fig. 9: H3-H4). This could mean that the older Jurassic flora persisted outside the Hartford basin, and that most of the pollen preserved in sediments did not come from the intrabasin flora. No pollen cones were available to test this hypothesis.
Flora C continues into the Portland formation at least 500 meters above the zone of igneous extrusives (Text-fig. 9, H5: Holyoke dam locality on Connecticut River). Some taxa drop out near the base of the Portland Formation, while at least one new taxon appears higher up in this assemblage. As lake beds higher in the Portland show fewer arid indicators and fern spores in them increase in number and diversity, Corollina torosa increases to dominance at the expense of C. meyeriana (Text-fig. 9). Flora C in the Hartford basin is replaced by flora D. The leafy shoots of flora D are much wider with larger leaves, which implies that they grew in a wetter climate (Text-fig. 8). By the stratigraphic levels of the Chicopee Falls and Agawam bridge localities (Text-fig. 9: H6-H7), all leafy shoots and leaves are large and fall into the form-genera Pagiophyllum and Elatides (e.g. Figs. 4-6). Brachyphyllum is either extremely rare or absent. Gypsum crystal casts, however, are still present in some brown mudstone units associated with the Agawam lake beds.
In the Deerfield basin, which is connected via a narrow neck to the northern end of the Hartford basin, a thick sequence of lacustrine strata (Turners Falls Sandstone) equivalent in age to the East Berlin Formation, Hamden basalt, and lower Portland Formation preserves a different flora sequence (Olsen et al., 1992). Instead of floral assemblages B, C, and D succeeding each other as they do in the Hartford basin, Floral Assemblage B, which is found in the oldest Jurassic strata (Fall River beds) underneath the Deerfield basalt, is replaced by Floral Assemblage D not far above the base of the Turners Falls Sandstone (Text-fig. 9; Olsen et al., 1992). Occasionally elements of Floral Assemblage C are found intermixed with Floral Assemblage D. However, fish faunas from the same layers producing the plants are dominated by semionotid holosteans. In contrast, redfieldiid subholosteans dominate coeval faunas in lacustrine strata of the Hartford basin. In addition, insect larvae (Mormolucoides: Fig. 39, m) are common in some shallow gray to black lake beds containing dinosaur footprints (Olsen et al., 1992). Similar larvae have not been found in the Hartford basin. Thus, the edaphic and climatic conditions that supported Floral Assemblage C in the Hartford basin appear to have been largely absent from the Deerfield basin at that time.
The Deerfield basin was apparently surrounded to its north and east by very high mountains, evidenced by the coarse angular conglomerate and bolder deposits around those margins (Bain, 1932). Floral Assemblage D could represent an allochthonous upland or even mountainous flora (i.e. mist forest) that enjoyed a cooler and much wetter climate than occurred in the Hartford basin at the same time (Cornet, 1989). Floral Assemblage C is replaced by assemblage D in the northern part of the Hartford basin as climatic conditions capable of supporting diverse fern populations returned. But later as the local climate in the Hartford basin deteriorated and lithofacies changed in the middle Portland Formation, assemblage D was replaced by Floral Assemblage E (Text-fig. 9).
Like Floral Assemblage B, assemblage E is dominated by the papillate cuticle type. Yet, leaf size was much larger, perhaps reflecting a greater gene pool of leaf characters for these new taxa to select upon than was the case for basal Jurassic cheirolepids. It is apparent that leaf size between floral assemblages D and E overlaps in the same way size overlaps between assemblages A and B. Similarly, a flora dominated by papillate cuticles replaces one dominated by smooth cuticles in both cases. Fern spores are about as common in palynofloras associated with both floras B and E, implying that climatic conditions may have been similar despite the existence of deeper lakes in the basal Jurassic Shuttle Meadow, Feltville, and Midland formations (Hartford, Newark, and Culpeper basins, respectively). Yet, the greater depth of these lakes was probably caused by increased basin tilting and greater subsidence along the deeper side of the half graben during the earliest Jurassic (Schlische and Olsen, 1990). Outcrops of basal Jurassic lake beds are mostly restricted to and are the thickest along that faulted margin. Much less tectonism is evident for the middle Portland Formation. The amount of water entering the Hartford basin in middle Portland and Shuttle Meadow times may have been similar. If it were spread over more of the basin and not ponded along one side, we can explain the extreme contrast between thin red lacustrine strata in the middle Portland Formation and thick gray to black lacustrine strata in the Shuttle Meadow Formation (Schlische and Olsen, 1990). Both types of lacustrine strata preserve organic material and palynomorphs, evidence that favors rapid sedimentation.
In summary, four successive floral assemblages dominated by cheirolepid conifers are recognized in the Newark Supergroup: A, B, C, and D (Text- figs. 8-10).
scale in millimeters
scale in millimeters
These assemblages range in age from Rhaetian to perhaps early Pliensbachian. The composition and character of each assemblage was affected by both evolutionary and climatic factors. Floral assemblages C and D existed in different areas at the same time, and reflect orographic and environmental differences, while floral assemblages A, B, and E represent temporal replacement as the regional climate cycled from wetter to drier and back to wetter conditions according to a pattern of Milankovich orbital periodicity (Olsen, 1986). Newark Jurassic floras are characterized below:
1) Floral Assemblage B represents the first wave of cheirolepid evolution after the Triassic/Jurassic-boundary event, and records a group of cheirolepids whose dwarf-shoots diverged radically from that of Hirmeriella. This group was not restricted to the Newark, but ranged as far north as Scotland in the basal Jurassic. The dominant pollen of this group and in most pollen cones was Corollina meyeriana (cf. Text-fig. 9).
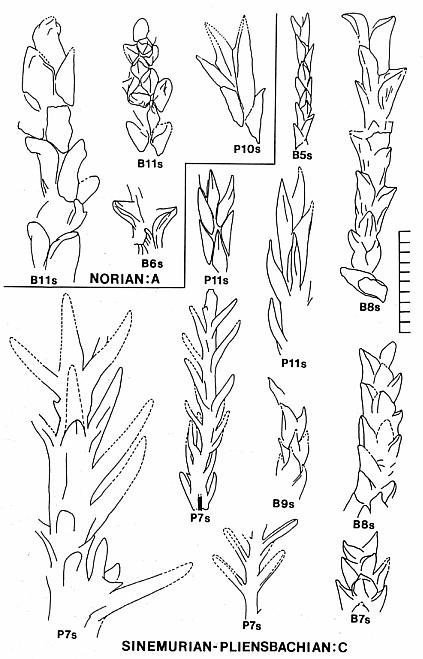
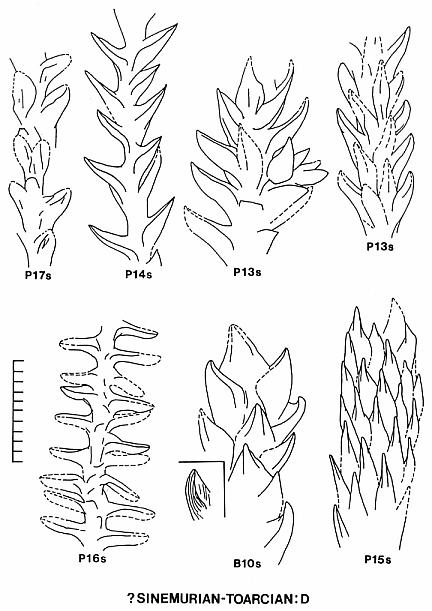
2) Floral Assemblage C represents a more conventional group of cheirolepid conifers dominated by Hirmeriella-type dwarf-shoots. This assemblage or group may have been adapted for physiological drought, living on intrabasin marginal-lacustrine soils containing evaporites ranging from calcite and gypsum to halite. The pollen of this group may have been a mixture of Corollina taxa, and included common C. torosa. Several leaf taxa illustrated in Text-figure 8 resemble in size, shape, and phyllotaxis some common European taxa: Hirmeriella muensteri (Schenk) Jung, Early Jurassic (B5s); Pagiophyllum maculosum Kendall, Middle Jurassic (P11s); Brachyphyllum scalbiensis Kendall, Middle-Late Jurassic (B8s); and Brachyphyllum desnoyersii (Brongn.) Kendall, Middle-Late Jurassic (Fig. 40, Deerfield basin, Horse Race). These similarites imply either that some taxa of assemblage C may be immigrants, and/or that some European species could be derived from Newark taxa.
3) Floral Assemblage D appears to have been an upland assemblage adapted more for humid climatic conditions and better drained soils (Text-fig. 10). It dominates most florules from Jurassic strata of the Deerfield basin. This flora migrated south into the northern part of the Hartford basin only when the lakes became much fresher than they had been when assemblage C dominated basin habitats. The dominant pollen of this group was C. torosa, but C. meyeriana is sometimes common in the palynoflora. Several leaf taxa illustrated in Text-figure 8 resemble in size, shape, and phyllotaxis common European taxa: Pagiophyllum insigne Kendall, Middle Jurassic (P14s); and Pagiophyllum araucarinum (Pomel) Saporta, Middle Jurassic (P13s; Figs. 4,5,10). P13s in the Hartford basin was named by Newberry (1888): P. simile (Newberry) Cornet and McDonald comb. nov. (see above). Dwarf-shoots from the Deerfield basin (Horse Race), which resemble Hirmeriella estonensis (Kendall) Cornet and McDonald comb nov., are also present (Text-fig. 7B). H. estonensis in England is associated with P. araucarinum (see Systematics section).
4) Floral Assemblage E replaced assemblage D as conditions in the basin once again became drier, but not as dry or hot as during the reign of assemblage C. Assemblage E also ushered in a period of evolutionary change and innovation with the appearance and dominance of a new cheirolepid subfamily, the Conanthoideae. The pollen of this group was a mixture of Corollina taxa, with C. torosa produced by Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov.
5) The large percentage of leaf and dwarf-shoot taxa in floral assemblages C and D that have close European look-a-likes from the Middle and Late Jurassic supports the palynomorph data for a maximum age of late Sinemurian-Pliensbachian. An older age would place biostratigraphy at odds with age determined from Milankovich-type cyclostratigraphy (Olsen et al., 1989: p. 11; Schlische and Olsen, 1990; Olsen et al., 1992: Fig. 3).
Floral evolution
While floral assemblages C and D represent the more conservative types of cheirolepid conifers found elsewhere, assemblages B and E were dominated by taxa that diverged considerably from the Hirmeriella "bauplan". Taxa belonging to assemblages B and E share a papillate type of cuticle, and lived in a similar fluvial-dominated setting, but they possessed radically different types of dwarf-shoot. The only commonality in their reproductive system was a non-inverted ovule that could not be pollinated directly, and a restricted opening to the pouch or ovary formed by the epimatium.
An exceptionally well-preserved dwarf-shoot was recovered from lacustrine siltstone just above the main fish-bearing lake bed of the Midland Formation, Culpeper basin, VA (Text-fig. 7A). Its preservation is due to charcoalification by heat during a forest fire. Numerous pieces of charcoal/fusinite were also recovered from the same layer. Heating caused the dwarf-shoot to shrink, but the enclosed seed did not shrink as much as the surrounding scale due to its larger mass. The micropylar region and micropyle (Text-fig. 7A, arrowhead) can be seen clearly on a seed with smooth, longitudinally striate sarcotesta. The abaxial vascularized attachment scar on the scale can be seen clearly, as can the adaxial opening to the pouch or ovary. The papillate cuticle on the dwarf-shoot is still evident, and is indicated by dot patterns. The scale possesses three distinct lobes, but no minor lobes are apparent, except perhaps as subdued flaps or ridges situated between the points of the lobes. This specimen was sent to a colleague through the mail in 1979 and was apparently lost, but the camera lucida drawings capture all critical characters of this specimen (originally illustrated in Cornet, 1977).
Aside from the reduced number of lobes on the dwarf-shoot, which is important for documenting a reduction trend in the family, the seed is clearly not inverted. It is highly unlikely that the seed became rotated during charcoalification, because the shrinking scale left torn fragments perched in positions on the seed that can be explained only if orientation remained essentially unchanged. Realignment of that fragment by its contact margins re-orients the seed in a vertical position with micropyle facing distally, not apically as it does in Hirmeriella muensteri (Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991). There is also no evidence of an extended micropylar nipple as in H. muensteri and no external indication of a pollen chamber (the Cheirolepidiaceae appear to lack a pollen chamber: Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991).
A new type of dwarf-shoot was recovered from coeval strata of the Feltville Formation, Newark basin, NJ (Text-fig. 7C; Fig. 45). This scale possesses a single distally oriented seed. A single vascular trace enters the base of the dwarf-shoot and divides, sending two branches laterally and one into the base of the seed. Part and counterpart to this specimen show that the seed was completely enclosed by dwarf-shoot cuticles with no clear evidence of a proximal opening. The resemblance of this dwarf-shoot to the scale/bract complex of Araucaria is apparent, implying that the Araucariaceae might be derived from the Cheirolepidiaceae. That interpretation was also suggested by Clement-Westerhof and Van Konijnenburg-Van Cittert (1991).
Like the Cheirolepidiaceae, the Araucariaceae did not become abundant in world floras until the Jurassic. Significant differences in pollen morphology between Araucariacites and Corollina argue for a Late Triassic age of their common ancestor, if the Cheirolepidiaceae and Araucariaceae are sister taxa. The oldest accepted araucarian pollen is Early Jurassic (Filatoff, 1975), although Araucariacites may extend back to the Norian (de Jersey and Raine, 1990; Cornet, 1993).
Another type of dwarf-shoot found at Horce Race (Turners Falls Sandstone) in the Deerfield basin, MA, shows three developed lobes and two reduced lobes between them (Text-fig. 7B). This scale resembles Hirmeriella estonensis Cornet and McDonald comb. nov. from the Middle Jurassic of England (Kendall, 1952), and is associated with common leafy shoots that resemble Pagiophyllum araucarinum, the probably leaf of A. estonensis. H. estonensis comb. nov. was originally placed by Kendall (1952) in Araucarites as a possible member of the Araucariaceae. Several araucarian pollen taxa are found in the Corollina torosa palynoflora (Text-fig. 9; Cornet and Traverse, 1975) that characterizes the interval where these plant fossils were found: i.e. Araucariacites australis, A. punctatus (Fig. 71), A. fissus (Fig. 67), Callialasporites trilobatus, and C. dampieri. Thus, the possibility of an araucarian affinity for some cheirolepid-like dwarf-shoots is raised.
Derivation of H. estonensis from the basal Jurassic dwarf-shoots described above would imply that the ovule/seed is not inverted. It is inverted in Araucaria and Hirmeriella. If the Araucariaceae were derived from the Cheirolepidiaceae, another cheirolepid lineage with an inverted ovule had to be ancestral. The dwarf-shoot in Text-figure 7B is probably derived from a Hirmeriella-type ovuliferous scale because of position and shape of its reduced (vestigial) lobes, but it is clearly younger than the oldest araucarian pollen in the Newark Supergroup, which first appears at the base of the Jurassic (Cornet, 1993). Yet, this dwarf-shoot taxon is older than H. estonensis (Sinemurian-Pliensbachian compared to Bajocian), implying that it could be related and that H. estonensis represents a further reduction of scale lobes.
Enter the Conanthoideae
The Conanthoideae represent a significant offshoot of the Cheirolepidiaceae: The two taxa in this subfamily described above demonstrate a novel experiment in pollination biology. Morphologically, the dwarf-shoots show an increase in number of scales from three to six. Understanding how this could happen requires an understanding of how the ovary and epimatium formed in the first place. In the case of Hirmeriella kendalliae, there are two ovules/seeds per dwarf-shoot (Text-fig. 7E). Each ovule appears to be encapsulated by its own dwarf-shoot cuticle. For that to happen, the area around each ovule primordium must have become invaginated into the scale primordium separately, carrying with it its immature ovule. In the case of Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov., this invagination apparently continued distally into the basal part of the central lobes as these lobes began to develop. The invagination process was probably responsible for deforming the shape of those lobes from lens or trapezoid in cross section to round or oval. Such a developmental process, if it occurred as described, would contrast strongly with the way in which the angiosperm ovary is formed.
The two species, Conanthus hystrixipinus and Conanthopsis hartfordensis, show differing degrees of development in lobes. The strong resemblance of C. hartfordensis to the Hirmeriella "bauplan" allows us to recognize and understand how this new scale morphology may have evolved. Clement-Westerhof and Van Konijnenburg-Van Cittert (1991) identify the two large lateral lobes on the dwarf-shoot of Hirmeriella muensteri as lobes, and note how they join the scale laterally for most of its length (Text-fig. 7D). The lateral lobes on Conanthus (incl. Conanthopsis) do the same thing, but it takes two lobes rather than one to cover the same distance (Text-figs. 3.3 and 3.5, shadded). The central lobe of Hirmeriella is replaced by two stigma-like lobes, and the number of lobes in the adaxial row has increased also by doubling compared to the hypothetical ancestral condition represented by Text-figure 3.1. Furthermore, the dwarf-shoot in Text-figure 3.2 seems to be intermediate in size and morphology, leading us to think that the dwarf-shoot of Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov. is a highly modified derivative of the basic Hirmeriella-type dwarf-shoot (cf. Text-figs. 3.1-3.5). That derivation, however, would seem to exclude the more simple and reduced dwarf-shoot types in the Newark basal Jurassic from being ancestral. That is because immigration of taxa from other areas is considered more likely than reversal of trends towards simplification in endemic taxa, and because immigration of exotic spore taxa (e.g. Staplinisporites caminus), which are more common in European paleofloras, has been documented.
Although no clear evidence is available to show how the ovule was oriented in the ovary, its orientation can be inferred from indirect evidence:
First, the seeds of Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov. are ovoid to round in shape, showing no clear area where the micropyle existed. There is no lateral attachment scar on the dispersed seed associated with one dwarf-shoot (Figs. 33-34), but there is a dimple within the seed perimeter offset to one side. If the dimple represents either the hilum or micropyle, it is clearly not oriented to one side or end as in Hirmeriella muensteri (Text-fig. 7D; Clement-Westerhof and Van Konijnenburg-Van Cittert, 1991). That would imply a modification in shape from the Hirmeriella-type ovule. A similar seed morphology is present in Conanthopsis hartfordensis Cornet and McDonald gen. et sp. nov. (Text-fig. 3.3).
Second, the cuticle is papillate like that of basal Jurassic cheirolepids in Floral assemblage B. If Conanthus is related to this group, and this group is characterized by non-inverted ovules (see discussion above), then it follows that the ovules of Conanthus (and Conanthopsis) were not inverted either. Derivation of Conanthus from the Brachyphyllum scottii group is weakened by dwarf-shoot morphology (see above), but they could represent sister taxa that diverged before any dwarf-shoot reduction began. Both taxa, however, represent significant deviations from the conventional Hirmeriella "bauplan".
Third, the development of specialized distal lobes on the dwarf-shoot for catching pollen and transmitting pollen tubes implies a strong selective force operating to bring micropyle and pollen closer together. Pollen germination on the dwarf-shoot has been inferred by others, and that mechanism apparently worked well when the micropyle was directed towards the proximal opening or ovary suture. It is possible that pollen tubes grew from the stigma-like lobes to the base of the ovary, or that they entered the chalazal end of the seed. But if either process operated successfully in Conanthus, there would be no need for specialized lobes. Shifting the hilum from a distal to more medial position, i.e. closer to the vascular traces entering the scale from the bract, may have been enough of a change to cause a similar shift in micropylar position. These three areas of inference suggest that the micropyle was situated close to the bases of the stigma-like lobes, and that it was not close to the proximal suture.
In concert with a shift in pollination strategy of the dwarf-shoot is a shift in developmental strategy of the female cone. Instead of the cone elongating gradually, and retaining its basic ovoid shape through development, the cone axis did not elongate as rapidly as did the sagitate bracts. This change in development produced an immature stage that resembles more a flower than a cone with its bracts organized in a rosette pattern (Text-figs. 3A, 3B; Figs. 21-24). No evidence of dwarf-shoot lobes can be detected at this stage. As the cone axis elongated, stigma-like lobes belonging to the dwarf-shoots appeared lateral to the bracts (Text-figs. 3D, 3E; Figs. 26, 31-32). As the cone continued to enlarge and elongate, additional lobes appeared (Text-fig. 4B). At maturity, the full complement of dwarf-shoot lobes is evident (Text-fig. 4C; Figs. 15-20).
The staggered development of bracts and dwarf-shoots implies that dwarf-shoot organs were delayed in development for a reason. The similarity of immature cones to flowers and mature cones to strobili is a strategy employed by a number of primitive angiosperms: i.e. Magnolia and Liriodendron to mention a couple classic examples. The delayed development of ovules/seeds, based on observations of amounts of carbonaceous compression material at each stage of cone development, implies that fertilization may have occurred before the ovules developed much nucellar food reserve. Either that or the pollen remained dormant on the scales until ovules were receptive, and the stigma-like lobes may have served a function in prolonging pollen viability. Clearly, female cones were immature at the time of pollen release (e.g. Fig. 55). Male cones at maturity somewhat resemble their female counterparts in having a tight rosette pattern of microsporophylls (compare Text-figs. 4A and 4D). Convergence in size and form at time of pollination implies that size and form were important. Biological vectors, such as beetles, might be the common denominator and reason. Various types of beetle elytra are common in the plant-bearing shales.
The difference in bract morphology between male and female cones may have served a function also, since elongate pointed bracts on the male cone would have discouraged insect visits, while their evolution on the female cone would have served to protect immature dwarf-shoots and helped to comb pollen off visiting insects. The sagitate shape of the female bract is an evolutionary innovation, since the basic bract morphology of Hirmeriella is oblate and broad with hardly any apical point. Attraction of insects between male and female cones was probably aided by odoriferous resins and/or a nectar-like food source. Resins are preserved within the ovary and stigma-like lobes (see Description with interpretation above).
Finally, mature dwarf-shoot morphology of Conanthus hystrixipinus is radically different from that of other cheirolepids, indicating another change in reproductive strategy from that of Hirmeriella. The helicopter-type diaspora would have increased seed dispersal distance, allowing this taxon to migrate more rapidly than other conifers. Its elongate leaves increased its growth potential and may have allowed it to mature faster in a riparian habitat. All these novel characters probably enabled C. hystrixipinus to dominate floodplain environments in the Hartford basin. This taxon is the first cheirolepid to qualify morphologically as a candidate for determinant (non-incidental) entomophily. We think we have discovered an evolutionary achievement in coniferalean reproduction and growth rate that rivals that of primitive arborescent entomophilous angiosperms.
What happened to Conanthus later in the Jurassic is not known, but those interested in angiosperm evolution should look for evidence of related cheirolepids in younger Jurassic and Cretaceous strata. Any argument put forth to explain the rapid radiation of angiosperms in the mid-Cretaceous needs to consider the implications of such a conifer in the Early Jurassic. There has been much speculation as to why angiosperms did not undergo their main radiation before the Cretaceous if they had evolved by the Late Triassic (Lidgard and Crane, 1988; Doyle and Donoghue, 1993; Cornet , 1993b). Indirectly Conanthus may give us understanding about assumptions regarding the competitive advantages of a primitive angiosperm-type reproductive strategy. Clearly, we had a gymnosperm lineage in the Jurassic that was doing things more aggressively in inland riparian habitats, things that previously had been thought to be reserved exclusively for angiosperms.
The distinct possibility arises that mid-Cretaceous angiosperms such as Archaeanthus linnenbergeri Dilcher and Crane (1984), and Lesqueria elocata Crane and Dilcher (1984) evolved a Conanthus-like strobiloid strategy (i.e. many spirally-arranged reproductive units), because insect vectors already existed that effectuated pollination for Conanthoideae in the Cretaceous (and could do the same for any plant that provided similar recognizable resources). The reason cheirolepidaceous members of the Conanthoideae have not yet been described from the Cretaceous may not be due to their absence (e.g. the unusual cheirolepid pollen genus Dicheiropollis may be related), but due to a scarcity of comparable data on upland/inland Cretaceous floras. It is already known that insects select characteristics of reproductive structures, and thus mold floral evolution (e.g. the Orchidaceae). Consequently, magnoliid beetle-pollinated strobiloid flowers are not novel, but could be a molded copy of a pre-existing and successful conanthid reproductive strategy. Thus, any resemblance of early or primitive angiosperms to conifers may be an example of assisted convergence rather than one of phylogenetic derivation.
Lesqueria elocata
from Crane and Dilcher, 1984
Archaeanthus linnenbergeri
flower stage
from Dilcher and Crane, 1984
Archaeanthus linnenbergeri
fruit-cone stage
from Dilcher and Crane, 1984
Were these innovations partly responsible for the continued dominance of the Cheirolepidiaceae in the Jurassic? Entomophily appeared after anemophilous cheirolepids became dominant; is there any parallel with angiosperms? Does the fluvial non-halophyte habit and habitat of Conanthus have significance with regard to the types of cheirolepids normally found in marginal marine and coastal deltaic environments? Is it possible that the more primitive cheirolepids with anemophilous pollination dominated estuarian salt marshes and bordered hardwater inland lakes containing evaporites (cf. Upchurch and Doyle, 1981), while the more derived and advanced entomophilous cheirolepids dominated upland and riparian habitats of inland floodplain valleys and around freshwater lakes? Could the persistence of cheirolepids in marine-deltaic environments of the mid-Cretaceous represent their last stronghold, while competition in the uplands was being won by angiosperms? Could the dominance of primitive magnoliid angiosperms in Early Cretaceous coastal habitats reflect a similar retrograde distribution of evolutionary characters?
Consider the paleoequatorial west African and east South-American rift basins with sequences capped by marine evaporite deposits, and the gradual replacement of Dicheiropollis, Corollina, and Alisporites by Clavatipollenites, Stellatopollis, Retimonocolpites, Lilliacidites, and Tricolpites in the Barremian-Aptian part of the Cocobeach fluvio-lacustrine sequence of the Gabon and Congo basins (Doyle, Biens, Doerenkamp and Jardiné, 1977; Jardiné and Magloire, 1965). This replacement began in equatorial land-locked rift valleys and then spread northward and southward into coastal facies of marine basins (Retallack and Dilcher, 1981). One could infer from this pattern that angiosperm competition with cheirolepids began in riparian environments like that dominated by Conanthus, and then spread to inland brackish lagoons and coastal habitats (e.g. Santana Fm., Araripe basin, Brazil: Crane and Maisey, 1991). Because angiosperms were able to provide greater floral diversity and genetic variation than conifers, insect coevolution proceeded at a faster pace with angiosperm, allowing them to successfully compete against and eventually replace cheirolepid conifers in all of their habitats. That replacement culminated in the extinction of the Cheirolepidaceae in the Cenomanian.
If you are looking for:
REFERENCES
Archangelsky, S., 1968. On the genus Tomaxellia (Coniferae) from the Lower Cretaceous of Patagonia (Argentina) and its male and female cones. J. Linn. Sec., 61: 153-165.
Alvin, K.L. and Pais, J.J.C., 1978. A Frenelopsis with opposite decussate leaves from the Lower Cretaceous of Portugal. Palaeontol., 21: 873-879.
Alvin, K.L., Spicer, R.A. and Watson, J., 1978. A Classopollis-containing male cone associated with Pseudofrenelopsis. Palaeontol., 21: 847-856.
Barnard, P.D.W., 1968. A new species of Masculostrobus Seward producing Classopollis pollen from the Jurassic of Iran. J. Linn. Sec., 61: 167-176.
Burger, D., 1965. Some new species of Classopollis pollen from the Jurassic of the Netherlands. Leid. Geol. Meded., 33: 63-69.
Boltenhagen, E., 1973. Quelques espèces du genre Classopollis (Pflug) Reyre du Crétacé Supérieur du Gabon. Rev. Micropaléontol., 16: 205-213.
Clement-Westerhof, J.A. and Van Konijenburg-Van Cittert, J.H.A., 1991. Hirmeriella muensteri: new data on the organs leading to a revised concept of the Cheirolepidaceae. Rev. Palaeobot. Palynol., 68: 147-179.
Cornet , B., 1977. The palynology and age of the Newark Supergroup. Ph.D. diss., Pennsylvania State Univ., 505 pp.
Cornet , B., 1989. Jurassic conifer assemblages and their paleoclimatic implications. In: P.E. Olsen, R.W. Schlische and P.J.W. Gore (Editors), Tectonic, depositional, and paleoecological history of Early Mesozoic rift basins, eastern North America. Amer. Geophys. Union, Washington, D.C., Field Trip Guidebook T351, pp. 116-118.
Cornet , B., 1993a. Applications and limitations of palynology in age, climatic, and paleoenvironmental analyzes of Triassic sequences in North America. In: S.G. Lucas and M. Morales (Editors), The nonmarine Triassic. New Mexico Museum of Natural History & Science Bull. No. 3, pp. 75-93.
Cornet , B., 1993b. Dicot-like leaf and flowers from the Late Triassic tropical Newark Supergroup rift zone, U.S.A. Modern Geology, 19: 81-99.
Cornet , B. and Olsen, P.E., 1985. A summary of the biostratigraphy of the Newark Supergroup of eastern North America with comments on early Mesozoic provinciality. In: R. Weber (Editor), III Congresso Latinoamericano de Paleontologia. Mexico. Simposio Sobre Floras del Triasico Tardio, su Fitogeografia y Paleoecologia, Memoria, pp. 67-81.
Cornet, B. and Olsen, P.E., 1990. Early to middle Carnian (Triassic) flora and fauna of the Richmond and Taylorsville basins, Virginia and Maryland, USA. Va. Mus. Nat. Hist., Guidebook No. 1, Martinsville, 87 pp.
Cornet, B., Traverse, A., and McDonald, N.G., 1973. Fossil spores, pollen, and fishes from Connecticut indicate Early Jurassic age for part of the Newark Group. Science, 182: 1243-1247.
Cornet, B. and Traverse, A., 1975. Palynological contributions to the chronology and stragigraphy of the Hartford basin in Connecticut and Massachusetts. Geoscience and Man, 11: 1-33.
Crane, P.R. and Dilcher, D.L., 1984. Lesqueria: An early angiosperm fruiting axis from the mid-Cretaceous. Annals of the Missouri Botanical Garden, 71(2): 384-402.
Crane, P.R. and Maisey, J.G., 1991. Fosil Plants. In Maisey, J.G. (ed.), Santana Fossils, an illustrated atlas. T.F.H. Publications, Inc., Neptune City, NJ, 414-421.
de Jersey, N.J. and Raine, J.I., 1990. Triassic and earliest Jurassic miospores from the Murihiku Supergroup, New Zealand. New Zealand Geol. Surv. Paleont. Bull. 62, 164 pp.
Dilcher, D.L. and Crane, P.R., 1984. Archaeanthus: An early angiosperm from the Cenomanian of the Western Interior of North America. Annals of the Missouri Botanical Garden, 71(2): 351-383.
Doyle, J.A., Biens, P., Doerenkamp, A. and Jardiné, S, 1977. Angiosperm pollen from the pre-Albian Cretaceous of Equatorial Africa. Bull Centres Rech. Explor.-Prod. Elf-Aquitaine, 1: 451-473.
Doyle, J.A. and Donoghue, M.J., 1993. Phylogenies and angiosperm diversification. Paleobiology, 19(2): 141-167.
Drinnan, A.N., Crane, P.R., Friis, E.M. and Pedersen, K.R., 1991. Angiosperm flowers and tricolpate pollen of Buxaceous affinity from the Potomac Group (mid-Cretaceous) of eastern North America. Amer. J. Bot., 78: 153-176.
Filatoff, J., 1975. Jurassic palynology of the Perth basin, western Australia. Palaeontographica B, 154: 1-113.
Francis, J.E., 1983. The dominant conifer of the Jurassic Purbeck Formation, England. Palaeont., 26: 277-294.
Harris, T.M., 1979. The Yorkshire Jurassic flora. V. Coniferales. British Museum (Natural History), London, 167 pp.
Hirmer, M. and Hörhammer, L., 1934. Zur weiteren Kenntnis von Cheirolepis Schimper und Hirmeriella Hörhammer mit Bemerkungen über deren systematische Stellung. Palaeontographica B, 79: 67-84.
Hughes, N.F., 1975. Plant succession in the English Wealden strata. Proc. Geol. Assoc., 86: 439-455.
Hughes, N.F., 1976. Palaeobiology of angiosperm origins: problems of Mesozoic seed-plant evolution. Cambridge Univ. Press, Cambridge, 242 pp.
Jardiné, S., Doerenkamp, A. and Biens, P., 1974. Dicheiropollis etruscus, un pollen caractéristique du Crétacé Inférieur Afro-Sudaméricain. Conséquences pour l'évaluation des unités climatiques et implications dans la dérivé des continents. Sciences Géologiques, Bulletin, Strasbourg 27: 87-100.
Jardiné, S. and Magloire, L., 1965. Palynologie et stratigraphie du Crétacé des bassins du Sénégal et de Côte d'Ivoire. Mem. Bur. Rech. Géol. Min., 32: 187-245.
Jung, W.W., 1968. Hirmerella münsteri (Schenk) Jung nov. comb. eine Bedeutsame Konifere des Mesozoikums. Palaeontographica B, 122: 55- 93.
Kendall, M.W., 1947. On five species of Brachyphyllum from the Jurassic of Yorkshire and Wiltshire. Ann. Mag. Nat. Hist., 14: 226-251.
Kendall, M.W., 1948. On six species of Pagiophyllum from the Jurassic of Yorkshire and southern England. Ann. Mag. Nat. Hist., 1: 73-108.
Kendall, M.W., 1949. On a new conifer from the Scottish Lias. Ann. Mag. Nat. Hist., 2: 299-307.
Kendall, M.W., 1952. Some conifers from the Jurassic of England. Ann. Mag. Nat. Hist., 54: 583-593.
Lidgard, S. and Crane, P.R., 1988. Quantitative analyses of the early angiosperm radiation. Nature, 331: 344-346.
Lorenz, J.C., 1988. Triassic-Jurassic rift-basin sedimentology, history and methods. Van Nostrand Reinhold Co., New York, 315 pp.
Malyavkina, V.S., 1949. Index of spores and pollen, Jurassic-Cretaceous. Trudy VNIGRI, n.s., n. 33, Gostoptekhizdat, Moscow, 138 p. (in Russian).
Médus, J., 1977. Des palynoflores de l'Infralias de Normandie (France). Geobios, 16: 647-685.
Meyen, S.V., 1987. Fundamentals of palaeobotany. Chapman and Hall, New York, 432 pp.
Miller, C.N., 1990. Stems and leaves of Cunninghamiostrobus goedertii from the Oligocene of Washington. Amer. J. Bot., 77: 963-971.
Morbey, S.J., 1975. The palynostratigraphy of the Rhaetian Stage, Upper Triassic in the Kendelbachgraben, Austria. Palaeontographica B, 152: 1-75.
Newberry, J.S., 1888. Fossil fishes and fossil plants of the Triassic rocks of New Jersey and the Connecticut Valley. U.S. Geol. Surv. Monographs, vol. 14, 95 pp.
Olsen, P.E., 1986. A 40-million-year lake record of Early Mesozoic orbital climatic forcing. Science, 234: 789-912.
Olsen, P.E., Schlische, R.W., and Gore, P.J. (Editors), 1989. Tectonic, depositional, and Paleoecological history of Early Mesozoic rift basins, eastern North America. Amer. Geophys. Union, Washington, D.C., Field Trip Guidebook T351, 174 pp.
Olsen, P.E., Fowell, S.J. and Cornet, B., 1990. The Triassic/Jurassic boundary in continental rocks of eastern North America; a progress report. Geol. Soc. Amer., Spec. Pap. 247: 585-593.
Olsen, P.E., McDonald, N.G., Huber, P., and Cornet, B., 1992. Stratigraphy and paleoecology of the Deerfield rift basin (Triassic-Jurassic, Newark Supergroup), Massachusetts. Dept. Geol., Univ. Massachusetts, Amherst, Contrib. No. 66, pp. 488-535.
Pettitt, J.M. and Chaloner, W.G., 1964. The ultrastructure of the Mesozoic pollen Classopollis. Pollen et Spores, 6: 612-620.
Pocock, S.A.J. and Jansonius, J., 1961. The pollen genus Classopollis Pflug 1953. Micropaleontology, 7: 439-449.
Poort, R.J. and Kerp, J.H.F., 1990. Aspects of Permian palaeobotany and palynology. XI. On the recognition of true peltasperms in the Upper Permian of Western and Central Europe and a reclassification of species formerly included in Peltaspermum Harris. Rev. Palaeobot. Palynol., 63: 197-225.
Retallack, G. and Dilcher, D.L., 1981. Arguments for a glossopterid ancestry of angiosperms. Paleobiology, 7: 54-67.
Reyre, Y., 1970. Stereoscan observations on the pollen genus Classopollis Pflug 1953. Palaeontology, 13: 303-322.
Reyre, Y., 1973. Palynologie du Mesozoique Saharien. Memoires du Museum National d'Histoire Naturelle, Series C, 27: 284 pp.
Schlische, R.W. and Olsen, P.E., 1990. Quantitative filling model for continental extensional basins with applications to Early Mesozoic rifts of eastern North America. J. Geol., 98: 135-55.
Srivastava, S.K., 1976. The fossil pollen genus Classopollis. Lethaia, 9: 437-457.
Taylor, T.N., 1981. Paleobotany - an introduction to fossil plant biology. McGraw-Hill Book Company, New York, 589 pp.
Taylor, T.N. and Alvin, K.L., 1984. Ultrastructure and development of Mesozoic pollen: Classopollis. Amer. J. Bot., 71: 575-587.
Taylor T.N. and Taylor, E.L., 1993. The biology and evolution of fossil plants. Prentice Hall, New Jersey, 982.
Taylor, T.N. and Zavada, M.S., 1986. Development and functional aspects of fossil pollen. In: S. Blackmore and I.K. Ferguson (Editors), Pollen and Spores: form and function. Academic Press, London, pp. 165-177.
Traverse, A., 1988. Paleopalynology. Unwin Hyman, Boston, 600 pp.
Traverse, A., Cornet, B. and Ames, H.T., 1975. A new look at the "Classopollis-Circulina" taxonomic-nomenclatural problem. Geoscience and Man, 11: 159-160.
Upchurch, R.C. and Doyle, J.A., 1981. Paleoecology of the conifers Frenelopsis and Pseudofrenelopsis (Cheirolepidaceae) from the Cretaceous Potomac Group of Maryland and Virginia. In: R.C. Romans (Editor), Geobotany II, Plenum Publishing Corp., New York, pp. 167-202.
Van Konijnenburg-Van Cittert, J.H.A., 1971. In situ gymnosperm pollen from the Middle Jurassic of Yorkshire. Acta. Bot. Neerl., 20: 1-96.
Van Konijnenburg-Van Cittert, J.H.A., 1987. New data on Pagiophyllum maculosum Kendall and its male cone from the Jurassic of North Yorkshire. Rev. Palaeobot. Palynol., 51: 95-105.
Clement-Westerhof and Van Konijnenburg-Van Cittert, J.H.A., 1991. New data on the fertile organs leading to a revised concept of the Cheirolepidiaceae. Rev. Palaeobot. Palynol., 68: 147-179.
Venkatachala, B.S. and Goçzan, F., 1964. The spore-pollen flora of the Hungarian "Kossen facies". Acta Geol. Acad. Sci. Hungary, 8: 203-228.
Wall, D., 1965. Microplankton, pollen, and spores from the Lower Jurassic in Britain. Micropaleontology, 11: 151-190.
Watson, J., 1977. Some Lower Cretaceous conifers of the Cheirolepidiaceae from the U.S.A. and England. Palaeontology, 20: 715-749.
Watson, J., 1982. The Cheirolepidiaceae: a short review. Phyta, Studies on living and fossil plants, Plant Comm. Vol., 265-273.
Watson, J., 1988. The Cheirolepidiaceae. In: C.B. Beck (Editor), Origin and evolution of gymnosperms, Columbia Univ. Press, New York, pp. 383-447.
Whalley, P.E.S., 1985. The systematics and palaeogeography of the Lower Jurassic insects of Dorset, England. Bull. Br. Mus Nat. Hist. (Geol.), 39: 107-189.
Witte, W.K., Kent, D.V. and Olsen, P.E., 1991. Magnetostratigraphy and paleomagnetic poles from Late Triassic-earliest Jurassic strata of the Newark basin. Geol. Soc. Amer. Bull., 103: 1648-1662.
(click on images for enlargements)
Plate 1 (Figs. 1-3)
Plate 1
Conanthus hystrixipinus Cornet and McDonald gen. et sp. novo
1. Associated branches with leaves; two branch orders preserved.
2. Branch system with four branch orders.
3. Enlargement of ultimate branch in Fig. 2, showing cluster of immature shoots (buds).Plate 2 (Figs. 4-12)
Plate 2
Comparison of leafy-shoots and branch systems of Early Jurassic cheirolepid conifers from the Newark Supergroup.
4. Pagiophyllum sp., Holyoke Power Co. dam locality, South Hadley Falls, lower Portland Fm., Hartford basin, Massachusetts (Virginia Museum of Natural History: YPM020); smooth cuticle type; P15s in Text-fig. 8.
5. Pagiophyllum simile (Newberry) comb. nov., Hadley Falls, lower Portland Fm., Hartford basin, Massachusetts (Redfield collection, VMNH); smooth cuticle type; P13s in Text-fig. 8.
6. Elatides sp., Chicopee Falls, lower Portland Fm., Hartford basin, Massachusetts (VMNH: YPM024); smooth cuticle type; P16s in Text-fig. 8.
7. Pagiophyllum sp., K-F quarry, middle Portland Fm., Hartford basin, Connecticut (McDonald collection, ); probable leaf and branch system of Conanthus hartfordensis Cornet and McDonald gen. et sp. nov.; papillate cuticle type.
8. Elatides sp., Chicopee Falls, lower Portland Fm., Hartford basin, Massachusetts (VMNH: YPM014); smooth cuticle type; P16s in Text-fig. 8.
9. Conanthus hystrixipinus Cornet and McDonald gen. et sp. nov., K-F quarry, middle Portland Fm., Hartford basin, Connecticut (McDonald collection, ); papillate cuticle type.
10. Pagiophyllum simile (Newberry) comb. nov., Horse Race, Connecticut River, middle Turners Falls sandstone, Deerfield basin, Massachusetts (Huber collection); smooth cuticle type; P13s in Text-fig. 8.
11. Pagiophyllum sp., Interstate 91 interchange to CT RTe. 9, upper East Berlin Fm., Hartford basin, Connecticut (VMNH); smooth cuticle type; P7s in Text-fig. 8.
12. Brachyphyllum scottii Kendall, East Round Top locality, lower Feltville Fm., Newark basin, New Jersey (Cornet collection, VMNH); papillate cuticle type; B4p in Text-fig. 8.Plate 3 (Figs. 13-14)
Plate 4 (Figs. 15-20)
Plate 5 (Figs. 21-26)
Plate 6 (Figs. 27-32)
Plate 7 (Figs. 33-45)
Plate 8 (Figs. 46-56)
Plate 9 (Figs. 57-75)
Plate 9
Palynomorphs typical of the gray claystone palynoflorule, K-F Quarry, Suffield, CT, middle Portland Fm., Hartford basin; see Cornet and Traverse (1975) for further taxonomic details.
57. Corollina meyeriana, polar view, 30 mu diam.; massive wall structure.
58. Corollina murphyi, oblique view, 41 mu wide; large vermiculate intrabaculi; note equatorial striations.
59. Corollina torosa, equatorial view, 34 mu wide; faint small intrabaculi.
60. Corollina torosa, polar view, 28.5 mu diam.; faint small intrabaculi.
61. Corollina torosa cf. murphyi, polar view, 35 mu in diam.; large intrabaculi, intermediate between C. torosa and C. murphyi.
62. Pilasporites allenii, 29 mu and 35 mu diam.; note raised triradiate laesurae on specimen to left; compare with Corollina endexinal body.
63. Possible endexinal body to Corollina torosa, 25 mu diam.
64. Granamonocolpites sp., 26 mu long. .
65. Conanthus cuticle, stomatal apparatus, 91 mu across; note raised dark ridges around guard cells.
66. Corollina torosa, equatorial view, 35 mu diam.; note equatorial striations on lower half and texture of exposed endexinal body.
67. Araucariacites fissus, folded (normally round), 74 mu long; rents or germinal fissures run subparallel to long axis.
68. Todisporites rotundiformis, folded, 40 mu diam.
69. Cycadopites westfieldicus, 47 mu long.
70. Alisporites thomasii, left saccus folded, 61 mu long.
71. Araucariacites punctatus, 45 mu diam.
72. Todisporites rotundiformis, 44 mu diam.
73. Perinosporites cf. P. pseudoreticulatus, 47 mu diam.
74. Perinosporites cf. P. pseudoreticulatus, 41 mu diam.
75. Densoisporites sp., 44 mu diam.; triradiate laesurae barely visible.This web page was created on 30 October 2000.
Last modified on 06/10/2003.
© B.Cornet 2000.