Fossil Evidence for Root Parasites (resembling
Balanophoraceae) in a Late Triassic
Tropical Rift Basin, Central Pangaea
Bruce Cornet, Ph.D.
Abstract
The time of angiosperm origin still eludes botanists.
During the past four decades several hypotheses have been proposed. Two became so
supported by data and popular among paleobotanists that they were elevated to theories:
The Cretaceous Origin and Primary Radiation Theory and the Anthophyte Theory of Angiosperm
Origin, which extended angiosperm evolution back to the Early Mesozoic. Recently
molecular biology falsified the Anthophyte Theory, and provided additional support for
earlier molecular studies that angiosperms have been independent of extant gymnosperms
back to their roots in the Devonian and Carboniferous. Molecular data have also
caused a revolution in the theoretical phylogenetic hierarchy of angiosperms, changing
botanical concepts about the most primitive living angiosperms. Within the new
framework fossil plants such as Sanmiguelia lewisii from the Late Triassic of
Southwestern United States are enjoying renewed interest, whereas before they were
rejected because they conflicted with popular theories and now false evolutionary
trees.
One enigmatic fossil plant from a paleotropical Late
Triassic basin in Virginia went unnoticed for 30 years, because 1) the work of its
discoverer was not taken seriously by other paleobotanists, and 2) it was erroneously
described as an araucarian conifer, a taxon which is unremarkable in the Mesozoic fossil
record. Root holoparasites such as the pantropical Balanophoraceae are rejected by
most botanists as having anything to do with the habits of the earliest angiosperms, and
yet they may provide a partial answer to Darwin's "abominable mystery".
Now there is reason to reevaluate the Triassic fossils from a new perspective: evidence
for a root parasitic habit on swamp cycadophytes and tree ferns. Three of Bock's
fossil species are redescribed and compared to members of the family
Balanophoraceae. One compares with Langsdorffia
in basic inflorescence construction, although outwardly it resembles Ombrophytum and Lathrophytum;
the second is compared and contrasted with Lathrophytum,
but the third compares so closely with Lophophytum that
it could represent an example of convergent evolution. If these Triassic taxa are
indeed basal angiosperms, they would represent the oldest megafossil record yet (~230 mya)
of the subdivision Angiospermae, and would add a substantial wrinkle to the habits and
habitats of the first angiosperms.
Table of Contents
Introduction
Description and Distribution of Extant Taxa
Primaraucaria wielandii
Primaraucaria species 2
Triassiflorites grandifolia
Discussion with Comparison
Conclusion
References
Introduction
Several gymnospermous groups have been proposed as
ancestral to the angiosperms: Glossopteridales, Pteridospermales, and Gnetales, but
through recent molecular studies, all extant gymnosperms can be excluded, including the
Gnetales, which were the latest suspects in the falsified Anthophyte Theory of Angiosperm
Origin (Donoghue and Doyle, 2000). That leaves only those groups of gymnosperms
which are extinct: Glossopteridales and Pteridospermales (unrelated to the Cycadales).
Angiosperm
taxonomy is going through a major revision in evolutionary hierarchy based on the
falsification of old schemes by molecular data (e.g. Wolfe et al., 1989; Winter et al.,
1999; Doyle and Endress, 2000; Sanderson and Doyle, 2001). The Magnoliales, for
example, were once thought to contain basal angiosperms, while the Nymphaeales were
thought to be more derived than the Magnoliales. As recently as a decade ago the
Chloranthaceae, Schizandraceae, and Trimeniaceae were thought to contain basal
angiosperms, but now hold a more derived status than the Nymphaeaceae. With the
discovery of Archaefructus - an aquatic angiosperm - in possible latest Jurassic
strata containing fossils of feathered dinosaurs (Sun et al., 1999; 2002), coupled with
the presence of waterlily-like leaves in Barremian-Albian strata of Virginia (Hickey and
Doyle, 1977), genetic studies are being taken more seriously by paleobotanists and
taxonomists. As a consequence, phylogenetic trees have changed considerably (e.g.
Doyle and Endress, 2000; Doyle, 2001). And with that change the Triassic and
Jurassic data discovered and published by Cornet (1986, 1989a, 1989b, 1996), Cornet and
Habit (1992), and Hochuli and Feist-Burkhardt (2004) seem to fit much better than before
(see Why.htm).
On the one hand,
recently molecular data have identified Amborella trichopoda as the most
primitive living flowering plant (Stephens,
1999). On the other hand, the molecular relationships between the members of the
Balanophoraceae and their pantropical and subtropical world distribution imply that this
family arose prior to the complete breakup of Pangea (including Gondwanaland) during the
Early Cretaceous. Nickrent (2003) states under Phylogeny:
"Click HERE
to see a tree generated using nuclear small-subunit (18S) rDNA sequences from 11 of the
possible 17 genera of Balanophoraceae, with Amborella included as an outgroup. Note
the strong biogeographical nature of the tree, with the Neotropical taxa forming one clade
that is derived from within the Paleotropical grade. The association of Dactylanthus
and Hachettea follows traditional classifications (e.g. Dactylanthaceae sensu
Takhtajan 1997), however, the additional association with Mystropetalon
(Mystropetalaceae sensu Takhtajan 1997) is surprising. Despite the wide geographic
separation (S. Africa, New Zealand, and New Caledonia), these data suggest an ancient
southern hemisphere association that traces to ancestors on the Gondwanan landmass."
(Nickrent, 2003) http://www.science.siu.edu/parasitic-plants/tables/Table-holopars.html
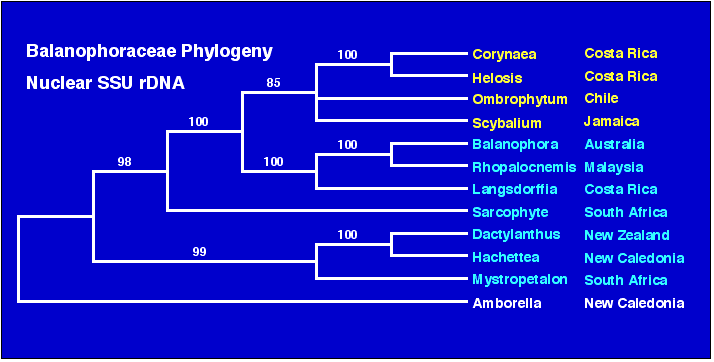
Image source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/images/Balanoph.phylo.gif
The relationship of the Balanophoraceae (and most
parasitic angiosperms) to other angiosperms is more equivocal, however. See Relationships of Parasitic Flowering Plants at http://www.science.siu.edu/parasitic-plants/Relation-Flowering.html.
During his investigation into pre-Cretaceous fossils
that might have a bearing on the evolution of angiosperms, Cornet came across a group of
fossils discovered by Wilhelm Bock, an amateur self-taught paleontologist. His fossils
came from a tropical rift basin in Virginia containing a diverse flora of large-leaved
pteridosperms, cycadophytes, pteridophytes (including tree ferns), horsetails, and
lycopods. Unfortunately Bock did not have enough botanical training to recognize the
uniqueness of these fossils, and he forced them either into the family Cycadales or into
the family Araucariaceae. Because one species had a superficial resemblance to
brachyphyll shoots with attached conifer-like cones, and because his specimens were older
than previously reported Araucaria species from Gondwanaland, he lumped all the
specimens under the name Primaraucaria wielandii Bock. Bock published on
these fossils in several professional papers, the most comprehensive of which is The
American Triassic Flora and Global
Distribution (Bock, 1969).
A cursory examination of his numerous photographs will
reveal that his fossils have a number of distinctive characteristics in common with the
Balanophoraceae. It is also within the same strata that Cornet discovered the Crinopolles group
of angiosperm-like pollen with reticulate-columellate wall structure. What is
interesting about this chronologic and stratigraphic association is that both Crinopolles
pollen (Triassic) and Balanochoraceae pollen (extant) have overlapping ranges in
morphology and aperture condition (i.e. monosulcate, disulcate, zonasulculate,
trichotomosulcate, trisulcate, pentasulcate, tricolpate, and spiraperturate for
Crinopolles pollen, and monosulcate, disulcate, trisulcate, pentasulcate, tricolpate,
polyporate, and foraminate for Balanophoraceae pollen).
Four specimens of Primaraucaria
were obtained from the Philadelphia Academy of Natural Sciences, where Bock had deposited
them. In his first paper on Sanmiguelia lewisii,
Cornet (1986) describes his analyses of Bock's specimens. He repeats that analysis
below with additional comparisons to genera of Balanophoraceae. Most of the fruiting
heads are comprised of unisexual flowers bearing large stalked carpels, but a bisexual
flower with latrorse laminar stamens was also identified. It may contain reticulate
pollen of the Crinopolles type. Small elliptical seeds were found inside the
carpels; like the seeds of the Balanophoraceae, they lacked evidence for a testa.
Description and Distribution of
Extant Taxa
Two internet sources of information are extensively
referenced and quoted or linked in this section. One is from the web page, The
Parasitic Plant Connection, by Dan Nickrent at http://www.science.siu.edu/parasitic-plants/index.html,
and the other is from the web page, The Families of Flowering Plants, the
Balanophoraceae, by L. Watson and M. J. Dallwitz at http://biodiversity.uno.edu/delta/angio/www/balanoph.htm
(full reference given below). Important information and images are reproduced or
linked from those websites for focus and convenience, rather than providing only
links. None of the information in this section about extant taxa belongs to
the author of this website.
Ombrophytum subterraneum

Above image source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/images/Ombrophytum.JPEG
Below internet source: Nickrent 2003 at http://www.science.siu.edu/parasitic-plants/Balanophoraceae/
Balanophoraceae
|
| Genera Included: Balanophora, Chlamydophytum, Corynaea, Dactylanthus,
Ditepalanthus, Exorhopala, Hachettea, Helosis, Langsdorffia, Lathrophytum, Lophophytum,
Mystropetalon, Ombrophytum, Rhopalocnemis, Sarcophyte, Scybalium, Thonningia. Takhtajan
(1987) split Balanophoraceae into six families. Among these, molecular evidence supports
only the segregation of Cynomoriaceae. Until further evidence exists, I choose to retain
the above genera in a single family. |
| Habit: Fleshy, achlorophyllous holoparasites |
| Parasitism: Attaching to roots of trees and shrubs (rarely herbaceous plants)
by a structure called a tuber which may contain only parasite tissue or mixtures of host
and parasite. Plants often accumulating a waxy product called balanophorin. |
| Roots: The slender rhizomes (roots?) grow from the tuber and form haustorial
connections to the host roots they encounter. |
| Stem: Absent (aerial portions technically an inflorescence) |
| Leaves: Scaly, without stomata, spirally arranged |
|
| Image source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/images/ScybaliumDepressum.jpg |
| Inflorescence: Inflorescence bearing "stems" arise endogenously
within the tuber. Branches subtended by scaly, reduced, caducous bracts, which in some
genera are peltate or in others triangular or clavate. |

|
| Langsdorffia
hypogaea (Nee and Whalen 16864). Female (above) and male (below) inflorescences
both sectioned longitudinally. Photo by M. Nee taken 7/26/79
6 km west of El Junquito, Venezuela. |

|
| Corynaea
crassa. Habit of plant showing inflorescence emerging from the large tuberous
haustorium. Cloudforest habitat (ca. 10,000 ft.) in the Talamancas, Cuerci biological
station, Costa Rica. Photo by Kyle Williams. |

|
| Ombrophytum
sp.. Inflorescences. Photograph by Al Gentry (voucher 39680). Links
to Missouri Botanical Gardens Tropicos Image Library. |
| Plant Sex: Plants monoecious or dioecious |
Flowers: Often minute and numerous - some of the smallest
flowers in the angiosperms. Unisexual, monochlamydous, entomophilous. |
Calyx: Staminate flowers diverse with 3-4 (-8) distinct or basally
connate, valvate tepals with a stamen opposite each tepal. |
Corolla: Absent |

|
| Mystropetalon
thomii [DLN 4091]. Female (top) and male (bottom) flowers. The ring-shaped, white
elaisome that sits below the ovary can be seen enclosesd within bracts of the female
flower. (Note: the fruit/elaiosome is upside down). The elaiosome is harvested by ants
which disperse the one-seeded fruits. The male flowers contain two rather typical stamens
(as compared to other Balanophoraceae!) that are attached to the perianth (tepals). The
flower is then subtended by bracts and bracteoles. Photo by D. L. Nickrent. |
Androecium: With a tetrasporangiate, dithecal anther opening by
longitudinal slits. Stamens often very reduced, monothecal anther opening by a terminal
pore, sometimes coalescent to form a synandrium opening irregularly from bilocular, rarely
trilocular numerous transverse slits. |
Pollen: 3-5 colpate or 3-many-porate or inaperturate, variously
binucleate or trinucleate, the binucleate types associated with a wet stigma, the
trinucleate ones with a dry one. |

|
| Mystropetalon
thomii [DLN 4091]. Same plant as above, but excavated to show that all genets
arise from the same stem. Note the new shoots being formed. A curious finding was the
presence of liquid surounding the central stems. Is this produced by the parasite? What is
its function? Photo by D. L. Nickrent. |
Gynoecium: Carpellate flowers lacking a perianth or in 2 genera with
(2) minute tepals, these hypogynous and united into a cup in Mystropetalon.
Gynoecium of 2 or 3 carpels united to form a compound ovary with (2-3) distinct styles or
a single trifid style or sometimes the stigma sessile and discoid, or in Balanophora
the gynoecium pseudomonomerous and with a single undivided style. Ovary typically solid,
without a locule or an obvious placenta or ovule (containing 1 or 2 embryo sacs - ovules
very reduced). Early ontogenetic stages of the ovary sometimes showing a massive central
placental column that later fuses with the ovary wall. |
Ovule: Apparently absent - without recognizable nucellus or
integuments. |
Embryo, etc.: monosporic or bisporic. Endosperm development cellular.
One few-celled embryo develops in the central tissues of the ovary and is surrounded by
endosperm and a layer of sclerenchyma at maturity. |
| Fruit: A tiny, indehiscent, one-seeded achene. In Mystropetalon,
surrounded by the swollen perianth tube. Individual fruits sometimes swollen and
aggregated into a flesh multiple fruit. |
| Seed: Solitary with a very small, undifferentiated embryo embedded in the
endosperm. |
| Chromosomes: X = 8, 9, 12 and more. |
Distribution |
|
Image source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/ |
Below internet source: http://biodiversity.uno.edu/delta/angio/www/balanoph.htm
Credit: L. Watson and M. J. Dallwitz (1992 onwards). The Families of
Flowering Plants: Descriptions, Illustrations, Identification, and Information Retrieval.
Version: 14th December 2000. http://biodiversity.uno.edu/delta/.
Dallwitz (1980), Dallwitz, Paine and Zurcher (1993, 1995, 2000), and Watson and Dallwitz
(1991) should also be cited (see References).
| Balanophoraceae L.C. & A. Rich. |
| Including Dactylanthaceae (Engler) Takhtajan, Hachettiaceae Van Tiegh., Helosidaceae
(Heloseaceae) (Schott & Endlicher) Van Tiegh., Langsdorffiaceae Van
Tiegh., Lophophytaceae Horan., Mystropetalaceae Takhtajan, Latraeophilaceae
Leandro ex A. St.-Hil., Sarcophytaceae Horan. |
| Excluding Cynomoriaceae |
| Habit and leaf form. Herbs. Plants of very peculiar vegetative form; fungoid
(the above-ground parts constitute the inflorescence, which is remarkably fungoid in
appearance, pallid, brown, pink or purplish, bearing numerous flowers that are among the
smallest known. The underground parts, which are attached to the host root, may be the
size of a pineapple and are tuber-like in appearance, exhibiting scale-leaves in only one
genus. The inflorescence develops within the ‘tuber’, ultimately rupturing it
and exhibiting its remains as a ‘volva’ at the base). Leaves much
reduced (but subterranean only), or absent. Plants rootless (at least in the
normal sense); more or less succulent; totally parasitic. On roots of the
host (of trees). Annual to perennial (without chlorophyll). Leaves when present,
membranous. |
| Stem anatomy. Secondary thickening absent. Xylem with vessels, or without
vessels. Vessel end-walls simple. |
| Reproductive type, pollination. Plants monoecious, or dioecious. |
| Inflorescence, floral, fruit and seed morphology. Inflorescences with densely
crowded flowers. Flowers minute. |
| Perianth sepaline (sometimes, in male flowers), or vestigial to absent; when
present, 3–8 (lobed); when present, free, or joined. |
| Androecium 1–2 (in achlamydeous male flowers), or 3–8 (equalling and
opposite P). Androecial members free of one another, or coherent. Androecium exclusively
of fertile stamens. Stamens 1–8; often isomerous with the perianth. Anthers cohering,
or separate from one another; dehiscing via pores, or dehiscing via short slits; bilocular
to four locular to many locular; tetrasporangiate. Anther epidermis persistent. Anther
wall with no differentiation of an endothecium; of the ‘dicot’ type. Tapetum
probably glandular. Pollen grains aperturate, or nonaperturate; when aperturate,
(2–)3–5 aperturate, or 3–4(–5) aperturate; colpate, or porate, or
foraminate; 2-celled. |
| Gynoecium 1–2(–3) carpelled. The pistil 1–2(–3) celled. Gynoecium
syncarpous; synovarious; superior to inferior. Ovary 1–2(–3)
locular. Gynoecium stylate (usually), or non-stylate. Styles apical. Stigmas 1, or 2.
Placentation apical. Ovules 1 per locule; pendulous; without integuments. Embryo-sac
development Polygonum-type, or Allium-type. Antipodal cells formed, or not
formed; when formed, 1, or 2; not proliferating. Endosperm formation cellular. Endosperm
haustoria present. Embryogeny piperad. |
| Fruit non-fleshy; indehiscent; a drupe, or a nut.
The drupes with one stone. Fruit 1 seeded. Seeds endospermic; without
a testa. Embryo rudimentary at the time of seed release. |
| Seedling. Germination type inapplicable — cotyledons lacking. |
| Physiology, biochemistry. Not cyanogenic. Alkaloids absent (one species). |
| Geography, cytology. All but one sub-tropical to tropical. Pantropical. |
| Taxonomy. Subclass Dicotyledonae; Crassinucelli, or Tenuinucelli (?).
Dahlgren’s Superorder Balanophoriflorae; Balanophorales. Cronquist’s Subclass
Rosidae; Santalales. APG (1998) family of uncertain position at the highest group level.
Species 120. Genera 17; Balanophora, Chlamydophytum, Corynaea, Dactylanthus,
Ditepalanthus, Exorhopala, Hachettea, Helosis, Langsforffia,
Lophophytum, Mystropetalon, Ombrophytum, Rhopalocnemis, Sarcophyte,
Scybalium, Thonningia. |
| Illustrations. • Habit and technical
details (Scybalium). |
Below internet source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/
Below image source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/images/LathrophytumPec.jpg
| Lathrophytum - no
photos |
|
Lathrophytum peckoltii |
Above image caption |
| Lathrophytum
peckoltii. Illustration from Hansen (1980). Legend of Fig. 22. A-H,
Peckolt sn 1886 after Hansen (1976), I and K (Pereira
5645) original. A, habit of young specimen, tuber lost, inflorescence
covered by peltate parts of bracts X1; B, bract subtending female branch,
side view X6; C, the same, front view of pelta X6; D,
bract subtending male flower, from below X6; E, male flower, side view
X6; F, the same, from above X6; G, female branch, side
view X6; H, female flower, side view X17.5; I, habit of
flowering specimen with host root, tuber, volva lobes, lower female part of inflorescence
showing peltas of branches, upper male part with anthers openeed, some 30 flowers have
lost the anthers X1, four male flowers with anthers open, three anthers lost, seen from
distal end X2.5. |
| Lathrophytum
peckoltii. Illustration from Hansen (1976). Legend of Figure 1: a: tuber, volva
with lobes broken off, and lower part of stem, presumably old specimen (herb. M); b: stem
with volva-lobes, inflorescence still covered by bracts, younger specimen (herb. C); c:
longitudinal section of young specimen, inflorescence still covered by volva (herb. K); d:
same, upper part enlarged, lower part of inflorescence with female-secondary axes, upper
part with male-flowers; e: bract supporting male-flower, lateral view slightly from
behind; f: same, from below; g: male-flower, from above; h: same, lateral view; i: bract
supporting female-secondary axis, lateral view; k: same, from behind; l: same, front view;
m: female-secondary axis, lateral view, flowers removed in lower part, still in position
in upper part, covered outwards by the enlarged, peltate top part of branch; n:
female-flower with 2 styles; o: same, longitudinal section. - Enlargement; a-c: scale 5
cm; d-m: scale 5 mm; n, o: scale 1 mm. - Material: a, b, e-o: PECKOLT s. n., leg. 1886; c,
d: GLAZIOU s. n., leg. 1886. |
Below internet source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/
Below image source: http://www.science.siu.edu/parasitic-plants/Balanophoraceae/images/Lophophytum2.jpg
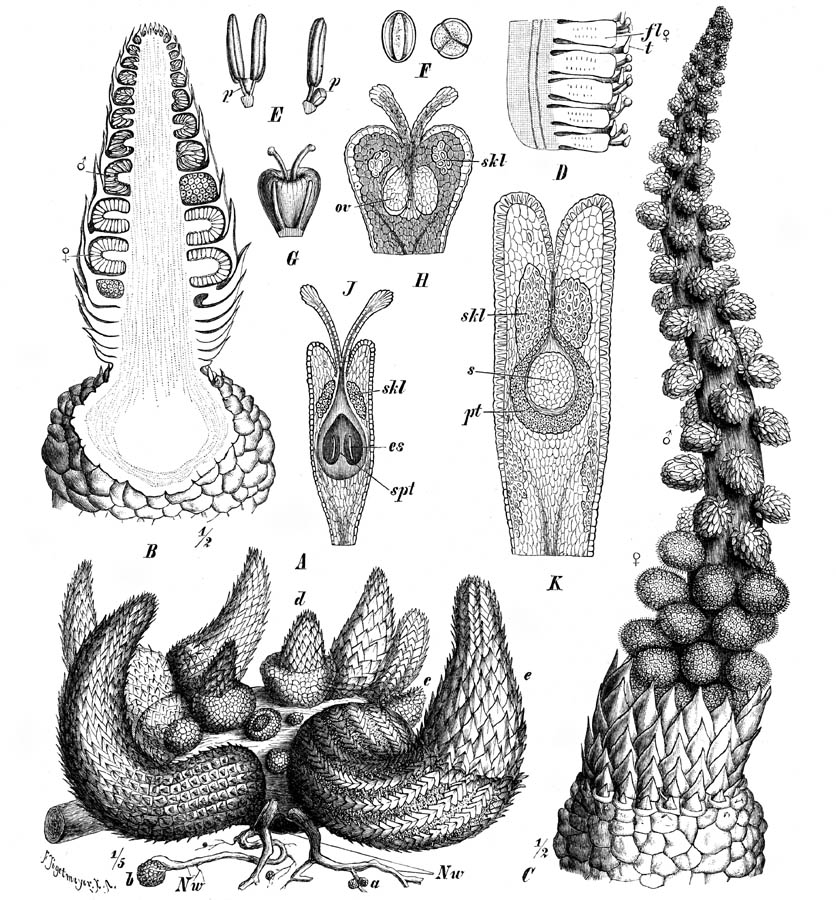
Above image caption |
| Lophophytum
mirabile. Inflorescence. Photo by Leppard [Leppard No. 445]. Slide no. 11934
archived at Kew. |
| Lophophytum
mirabile and L. leandri. Illustration from Harms (1894). Figure 161 legend,
translation from German. A-O Lophophytum mirabile Schott et Endl. A group of young
plants in different stages of the development, on a strongly swollen host root (Nw); a =
very young tubers; b = somewhat older; c tuber with the beginning of the development of
leaves. d = somewhat older stage of a tuber growing into an inflorescence, without
cataphylls at the lower part; e = older growing rhizomes with cataphylls covering also
basal parts. B longitudinal section through a young inflorescence, showing the shieldlike
stalked bracts and the individual spadices of the second order. C flowering plants, in
which the bracts have been shed - D part of a female spadix of L. leandri Eichl.,
showing the bracts of the flowers. - E-K L. mirabile. E male flower from the side
and from the front, with p = pistil vestige. F pollen (240/1). G female flower with two
staminodia. H ovary in longitudinal section, ov = ovule., skl = sclerenchyma group. J
longitudinal section of a somewhat older ovary, showing further advanced development of
the upper ovary rim, with ovules completely adhered to the ovary wall .; spt = the
septum-like extension of the placenta, es = embryosac. K longitudinal section of a fruit,
pt = endocarp, s = seed. (after Eichler.) |
| Lophophytum
weddelllii. Illustration from Hooker (1856). Fig. 1. Portion of section of male
inflorescence. Fig. 2. Portion of section of female inflorescence. |
Primaraucaria wielandii
Bock
This section is by the author, Bruce Cornet.
If you read what Bock (1969) says about P.
wielandii, his description seems as if it were taken from a botany manual on Araucaria.
But when you examine his pictures, they don't agree with what is known about Araucaria,
fossil and extant. For one, the male reproductive structures are not cones, but
flowers with perianth parts organized on an elongated inflorescence axis (spike).
Bock assumed that the adnate spirally-arranged bracts on the lower parts of inflorescence
axes could be compared to the brachyphyll leaves of conifers, but they are at least three
times the size of xerophytic araucarian leaves. Bock further assumed that silicified
tree trunks found in the youngest strata of the basin belonged to Primaraucaria,
even though this plant was found only in the oldest strata of the basin (the two horizons
are separated by as much as 6,000 feet of strata). It has since been demonstrated
that those tree trunks belonged to Glyptolepis-Pagiophyllum, a common
Late Triassic conifer of North America that contributed to the formation of the Petrified
Forest of Arizona. And if you examine actual specimens, dissect them, and prepare
macerations of bracts and reproductive organs as I have done, it will become apparent that
this was a non-woody herbaceous plant that lived in close association with swamp
vegetation, such as Macrotaeniopteris, with which it is commonly associated as a
fossil. Macrotaeniopteris had a leaf and venation that closely resembled a
small version of Musa.
Even after its angiospermous characteristics had been recognized (Cornet, 1986),
its rightful place in botany had to wait for the internet and for old concepts of basal
angiosperms to give way to new taxonomic hierarchies based on molecular data before such
an unusual plant could be compared, let alone accepted, as possibly the oldest (parasitic)
angiosperm yet recognized.
Primaraucaria was first found by Wilhelm Bock in the
1950's, and first named in 1954. It was found in shales of coal mine dumps at
Winterpock, Virginia. The coals from the Winterpock mine are found near the base of
the stratigraphic sequence in the Richmond basin of Carnian (Late Triassic) age (Cornet
and Olsen, 1990; Cornet, 1993). At the time this plant existed, the Richmond basin
was located just north of the paleo-equator in the same relative position the
Balanophoraceae occur today.
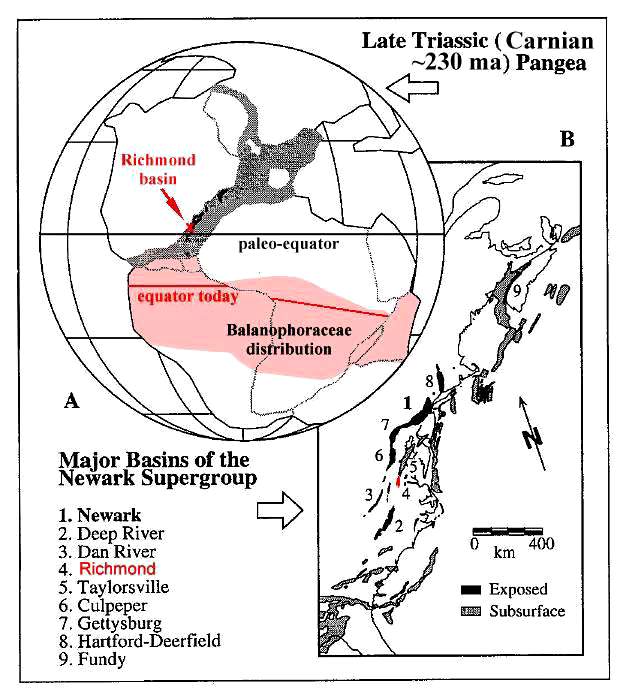
Figure modified from Olsen and Kent (2000).
Cornet (1986) had the opportunity to study four well-preserved specimens
identified by Bock as Primaraucaria. After careful examination, he
discovered that this small collection contained not one, but two distinct species based on
reproductive structures. One species (figs. A-C below) had a unisexual female
inflorescence that outwardly resembles the heads of Corynaea,
Lathrophytum, and Ombrophytum
of the Balanophoraceae, but differed internally. The second species (fig. D
below) had a bisexual inflorescence with apetallous multicarpellate female flowers
comprised of pedicelate ascidiate carpels on an elongate receptacle or axis, and laminar
stamens located below the female flowers on the inflorescence axis. Bock also
illustrates what may be the male flowers of the first species, which had elongate bracts
below an axis bearing male flowers with perianth elements.
Four specimens with labels (A, C, and D also have
impressions/compressions of Macrotaeniopteris leaves).
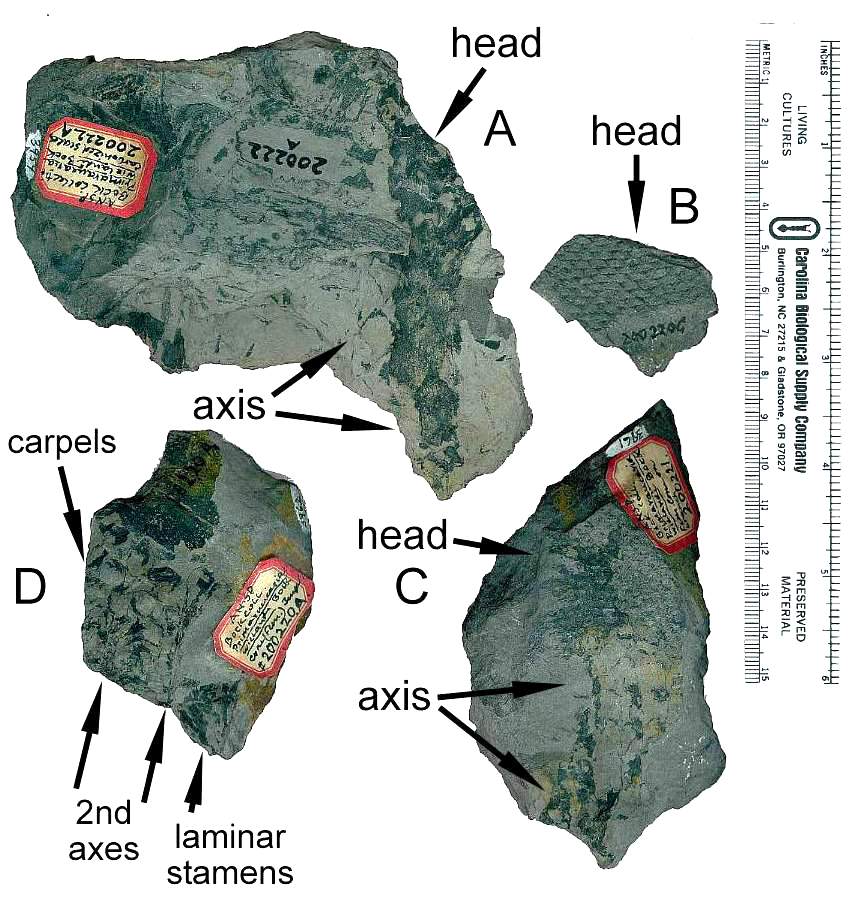
Above specimens from the Bock collection at the Philadelphia Museum of Natural History.
Below from Bock (1969: 313, figs. 534 and 541), showing both female (left) and male
(right) inflorescences that may belong to the same species. A possible second female
head can be seen in the lower right corner of figure 534, implying that multiple
heads-inflorescences were produced in close proximity to one another.
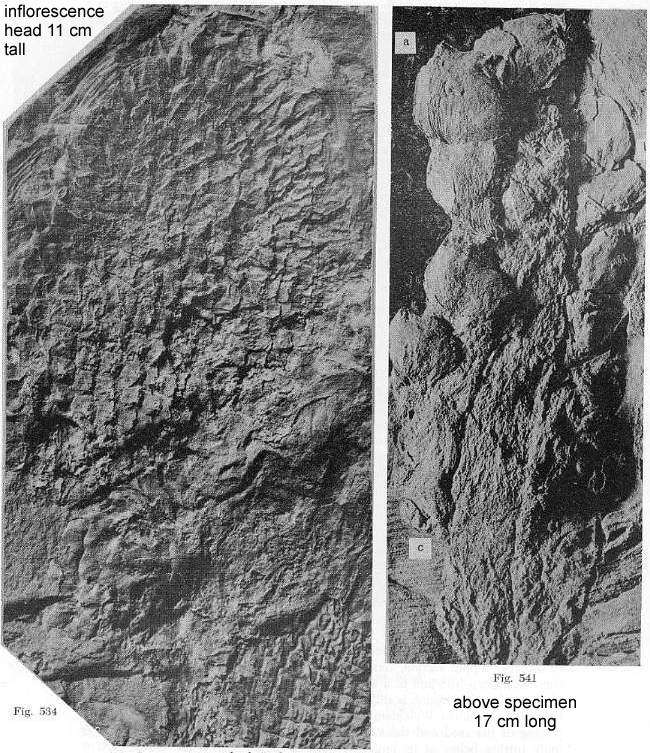
Another more complete specimen of the male inflorescence on the right (fig. 541) is
shown below (Bock, 1969: figure 536) so that the subtending whorl of elongate bracts can
be seen (cf. Langsdorffia).

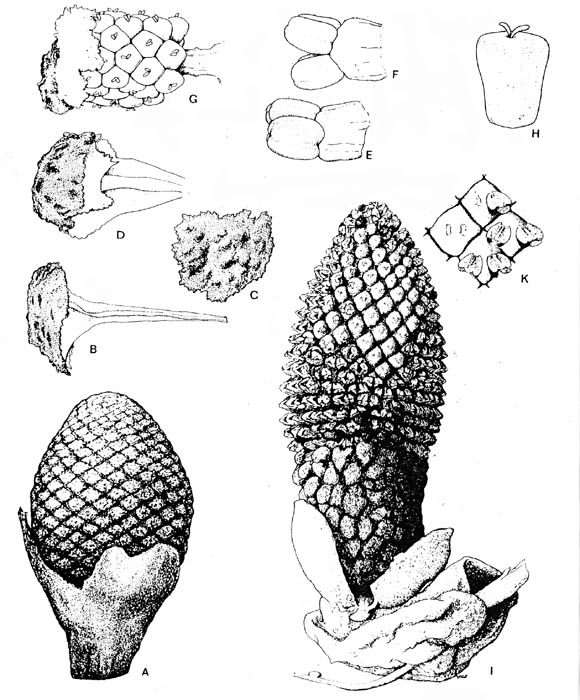
Plate 9 and caption from Cornet (1986).
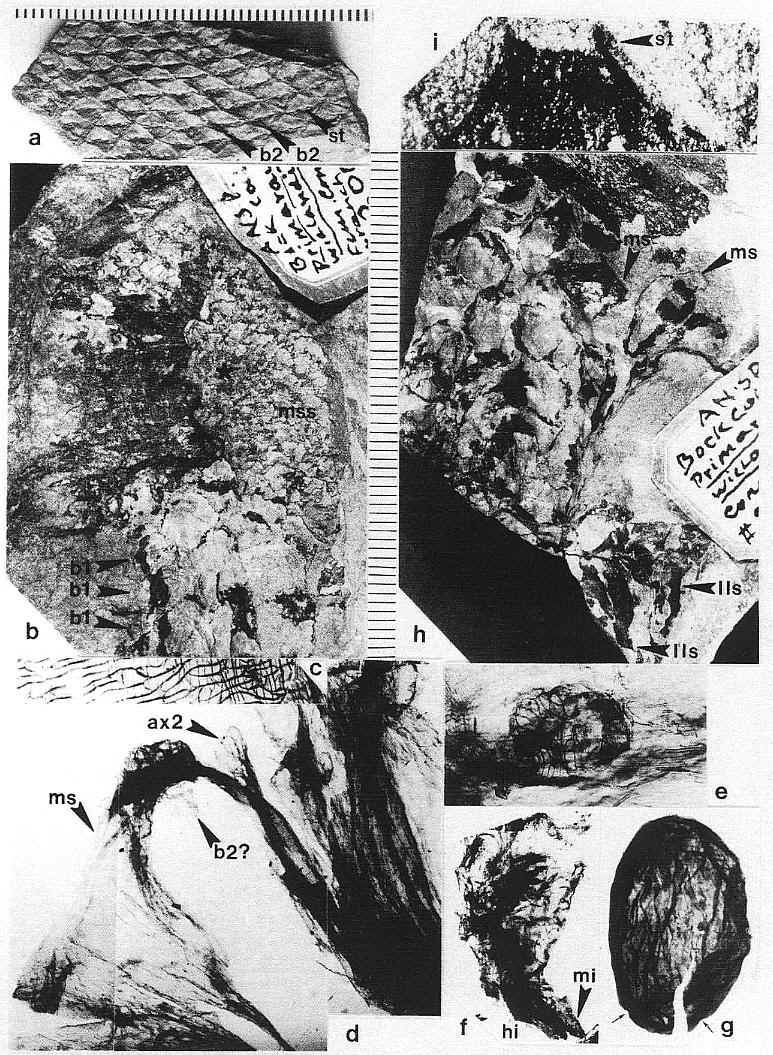
Plate 9,
figs. a-i. Zamiostrobus virginiensis Fontaine 1883 or Primaraucaria
wielandii Bock 1954 sensu lato, Productive Coal Measures, Richmond
Basin, VA. -a. TYPE A fruiting structure, ANSP #200220C (3953).
Impression of fruiting head with spiral phyllotaxis of megasporophyll subunits, each
showing folded impression of a subtending bract (b2) and an apical scar (st) to a
stigma-like process; scale in mm. -b. TYPE A fruiting structure, ANSP
#200221 (3961). Compression of a fruiting head (mss) terminating a long reproductive axis,
possessing numerous spirally arranged bract-shaped leaves (b1); * indicates position of
portion of compression removed for study (see figure 9a for a reconstruction); scale to
right in mm: x 2. -c. TYPE A fruiting structure: Paired cuticles of
subtending bract from specimen in fig. b (*); x 1,250. -d. TYPE A
fruiting structure: Main axis and base to one carpel-like megasporophyll subunit (ms),
showing diverging secondary branches (ax2) and possible base to subtending bract (b2?);
from * in fig. b (see figure 109); x 3.5. -e. TYPE A fruiting structure: Aborted,
250 um wide, anatropous ovule attached to cuticle of ovary wall; from * in fig. b; x 112. -f.
TYPE A fruiting structure: Immature, 2.6 mm long seed removed from an ovary, showing
micropyle (mi) adjacent to hilum (hi) and strongly recurved embryo body; from * in fig. b;
x 20. -g. TYPE A fruiting structure: Nearly mature, 2.8 mm long seed with
a thick wrinkled seed coat, showing only two small pits or depressions at one end and no
functional micropyle or obvious hilum scar; from * in fig. b; x 18. -h. TYPE B fruiting structure, ANSP #200220A
(3962). Compression of a fruiting head composed of numerous pedicellate carpel-like
megasporophyll subunits (ms), collectively subtended by large latrorse laminar stamens
(lls); see figures 9b and 10h); scale to left in mm: x 2. -i. TYPE B
fruiting structure: Distal end of a megasporophyll in fig. h, showing pore-like opening
(st) that may represent the base to a deciduous stigma; x 16.6.
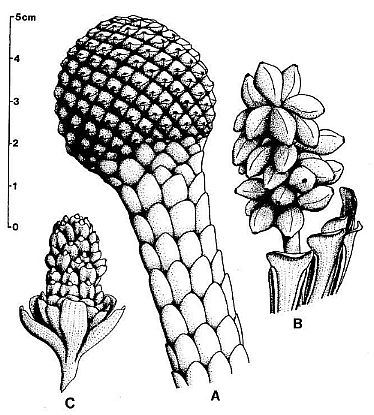
Figure 9. a-c. Zamiostrobus
virginiensis Fontaine 1883 or Primaraucaria wielandii Bock 1954 sensu
lato. Reconstructions of three different types of fruiting heads and
reproductive structures illustrated by Bock (1969) under P. wielandii. -a.
TYPE A. -b. TYPE B. -c. TYPE C. See text for further
descriptions; scale in cm: x 2. (http://www.sunstar-solutions.com/sunstar/evoltheo/slewisii.htm)
AD: Cut down your exam stress by using our latest PW0-105 braindumps and high quality 352-001 and ccna practice questions
demos. We provide updated 642-447
braindumps with 100% pass guarantee along with PMI
.
[11.24.14-17]
Primaraucaria wielandii Bock
See Bock (1969), pages 309-333 at www.sunstar-solutions.com/sunstar/Primarau/Bockindex.htm.
Habit Fleshy, non-woody (no coalified core as
occurs in woody plant compressions), typically found as inflorescence axes with scale
bracts terminating in an inflorescence head.
Parasitism One specimen of an inforescence axis
attached to a portion of a tuber - illustrated in Bock (1969: fig. 530).

Roots No evidence of roots found or recognized,
even though this plant could not have been transported far from where it grew due to its
size and shape, and its association with so many large intact Macrotaeniopteris
leaves.
Stem Absent (aerial portions technically part of an
inflorescence) based on the fact that attached leaves are largely devoid of stomata,
implying a lack of photosynthesis. One 2.4 cm wide scaly axis was found by Bock
(1969: fig. 529) showing a termination without a reproductive structure. That
termination is similar to an immature inflorescence axis of the Balanophoraceae (see Mystropetalon
thomii on Nickrent's website) in the process of pushing itself up through the soil
before developing a fertile head.
 For
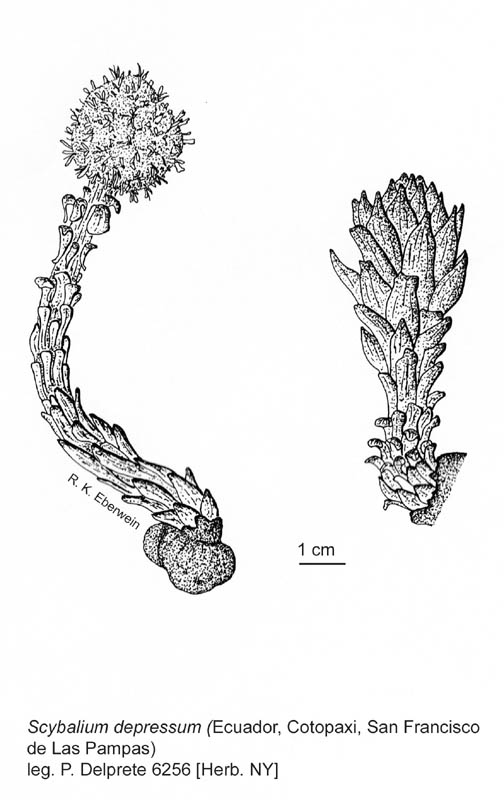
comparison see an immature inflorescence of Scybalium jamaicense.
For
comparison see an immature inflorescence of Scybalium jamaicense.
Because some axes found by Bock were as long as 14 cm (e.g. his figure
528), he assumed they were fallen branches from a tree. The scaly axis of Scybalium depressum, for example, grew to 9 cm in length
before terminating in a reproductive head. Some axes of Helosis cayennensis can grow to 10 cm before terminating in a
reproductive head.
Leaves Large, scaly, easily folded or wrinkled,
with rare stomata, spirally arranged. Stomatal structure variable, commonly closed
or occluded - nonfunctional. Epidermal hairs reduced or vestigial.
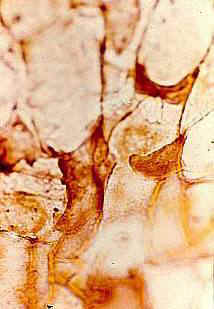
Inflorescence, floral, fruit, and seed morphology No
evidence of vegetative stems. Inflorescence-bearing axes appear to arise directly from a
tuber (see figure 530 above). Axes subtended by large, wide, scaly, flat (as in adnate)
bracts. In male inflorescences a secondary perianth-like development of bracts
present below the fertile portion of inflorescence axis - may have functioned as bud
scales.
Perianth Present only in male flowers, 1-2?
(lobed); isomerous with and joined to anthers.
Androecium The number of stamens and petals cannot
be accurately determined from Bock's (1969) illustrations, but they appear to be
few. One, possibly two petals can be detected; they conceal the stamens. Bock points
out an exposed anther in figure 541c above. Based on rare pollen found clining to
outer carpel cutile, pollen grains are simple monosulcate.
Gynoecium Unicarpellate. Carpels pedicellate with a
swelling or node where pedicel joins carpel, which may have been the attachment point for
vestigial perianth parts (see Plate 9 above and image below). Style and stigma
apparently dehiscent at maturity or lost during fossilization, leaving behind a circular
aperture at apex of carpel. Carpels 3-4 mm wide, about 9-10 mm long; length of pedicels
could not be determined.
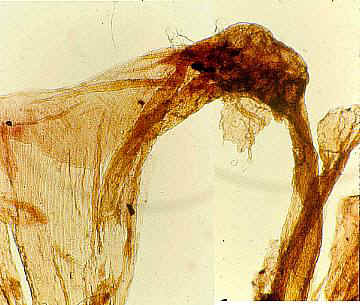
Ovules 3-4 per locule; pendulous; without integuments; ovule cutile
continuous with inner carpel cuticle.
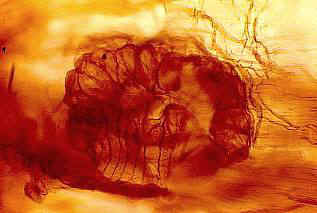
Suggested restoration of pedicellate carpels showing pendulous seeds.
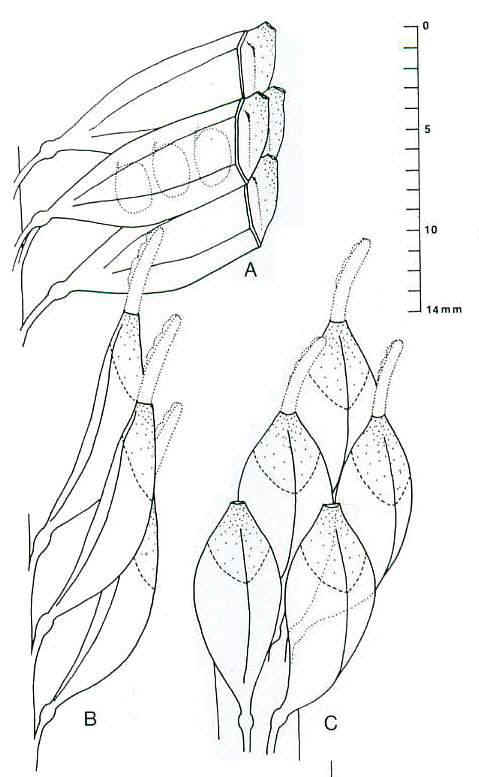
Plant sex Unknown, could be monoecious or
dioecious.
Flowers Unisexual, female naked (possibly reduced
monochlamydous).
Fruit Probably non-fleshy; indehiscent; a
compound multiple fruit, like a pineapple. Individual carpels contain 2-3
seeds. Seeds without testa; only a thickened
fleshy cuticle present. Embryo apparently rudimentary at the time of seed release
based on condition found in fruit. Thin sections through compression fossil show
seeds within multiple cuticles of carpels.
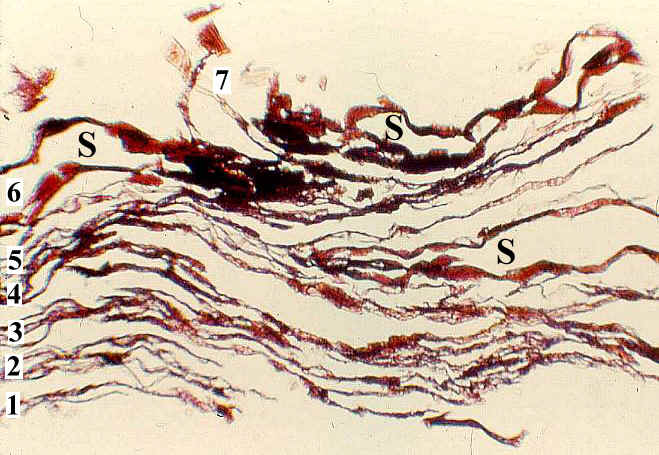
Seedling Germination type unknown — cotyledons
apparently lacking as in the Balanophoraceae.
Germinating seed of Dactylanthus taylorii
(Balanophoraceae) shown next to a seed of Primaraucaria (right - 2.8 mm)

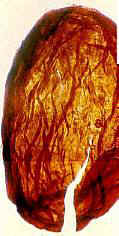
Image on left source (Nickrent, 2003): http://www.science.siu.edu/parasitic-plants/Balanophoraceae/
Primaraucaria species 2
The second species is based on only one specimen of a reproductive structure (see
labelled figure D above or enlargement below).
Inflorescence and floral morphology This species
differs from P. wielandii in that both male and female reproductive structures
are present on the same inflorescence. Either the inflorescence axis branches
(compound inflorescnece), with the secondary branches each bearing numerous unicarpellate
flowers, or the flowers are multicarpellate, with pedicellate carpels loosely arranged
along an elongate receptacle (depicted as a peltate bract in Sarcophyte
sanguinea, Lophophytum
mirabile and L. leandri, and Lathrophytum
peckoltii). At least 10 carpels per flower. Laminar latrorse stamens
subtend the ovuliferous part of the inflorescence (based on position and orientation).

Flowers Unisexual, dioecious.
Perianth Absent or unknown if independent of
anthers.
Androecium At least three laminar stamens preserved
on the one specimen on one side of the inflorescence. Anthers elongate, one on each margin
of tepaloid lamina; dehiscence slit longitudinal; indications of single chamber at
maturity. Pollen is still present inside the anthers, and awaits extraction,
preparation, and study.
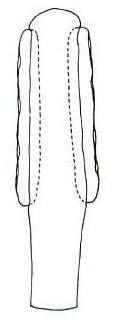
Gynoecium Apocarpous. Carpels pedicellate with a
circular aperture at the apex of the carpel. If existed, the style and stigma
apparently dehiscent at maturity or were lost during fossilization. Carpels elliptical in
shape; 1 cm long, 5-6 mm wide; pedicels about 1 cm long. No seeds could be detected
within carpels, and no attempt to remove and macerate a specimen was attempted.
Based on morphology and size, the carpels are similar in construction to P. wielandii,
and therefore probably contained multiple ovules/seeds.
Triassiflorites grandiflora
Bock
In addition to Primaraucaria, Bock (1969) describes
another fossil preserved only as an inflorescence. Bock relates that over many years
of collecting, he was able to recover 12 complete inflorescences of this species and 30
additional fragments. He was convinced that the close association with Macrotaeniopteris
leaves indicated an affinity with that plant, even though Primaraucaria was also
intimately associated with Macrotaeniopteris leaves (see above).
As with Primaraucaria, he interpreted Triassiflorites as a gymnosperm,
but in this case as a member of the Cycadales. Strangely, he described the
reproductive organs as "flowers" with perianth parts.
A close examination of the pictures provided by Bock (1969:
265), however, raise doubts as to its cycadalean affinity. Instead his pictures
reveal remarkable similarities with the Balanophoraceae genus Lophophyton.
Both the anthers and carpels compare remarkably well in size and shape to those
illustrated for Lophophyton (see above). The only significant differences
that can be detected are 1) the female secondary branches on a compound bisexual
inflorescence retained perianth parts (see Bock,
1969: 273), whereas in Lophophyton they do not, 2) the male secondary
branches were longer than those of Lophophyton, and contained many more anthers
(naked male flowers), and 3) there may have been more variation in the distribution or
number of male and female secondary branches per inflorescence, with some described as
being mostly male while others had male and female flowers mixed on the same secondary
branch. This is intuitively possible because female flowers were not naked (as in Lophophyton),
but were shielded from self-pollination by perianth parts.
Preservation (or the quality of his illustrations) is
not good enough to determine if Bock's interpretation of more than one female flower per
secondary branch is correct, or is the result of compaction and distortion of the
inflorescence during burial. Even though Bock distinguished between sterile bracts
subtending fertile secondary branches, overlap led him to think that the thousands of
biloculate anthers per branch were instead attached to the bracts. And yet in some
of his photographs and reconstructions he provides evidence for their separation and
distinction. In addition, Bock's reconstruction (Fig. 450 below) shows neatly
arranged "sporangia" on the anthers, but a close examination of his photographs
shows random "bumps", which might instead be remnant pollen grains inside pollen
sacs. Compare Bock's photographic plates below with illustrations of Lophophyton above:
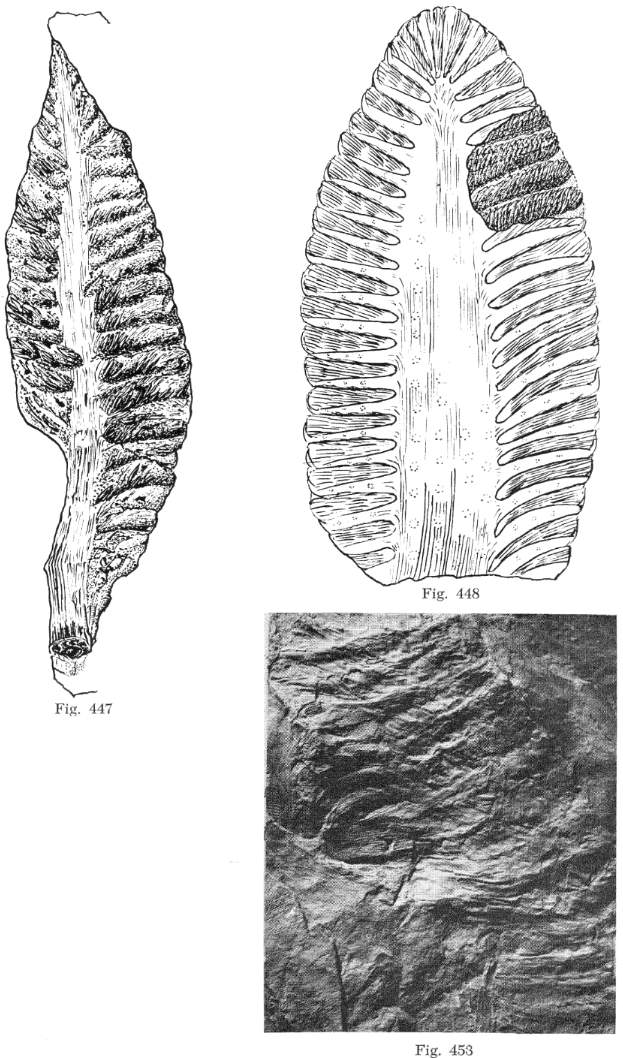
From Bock (1969: 271).
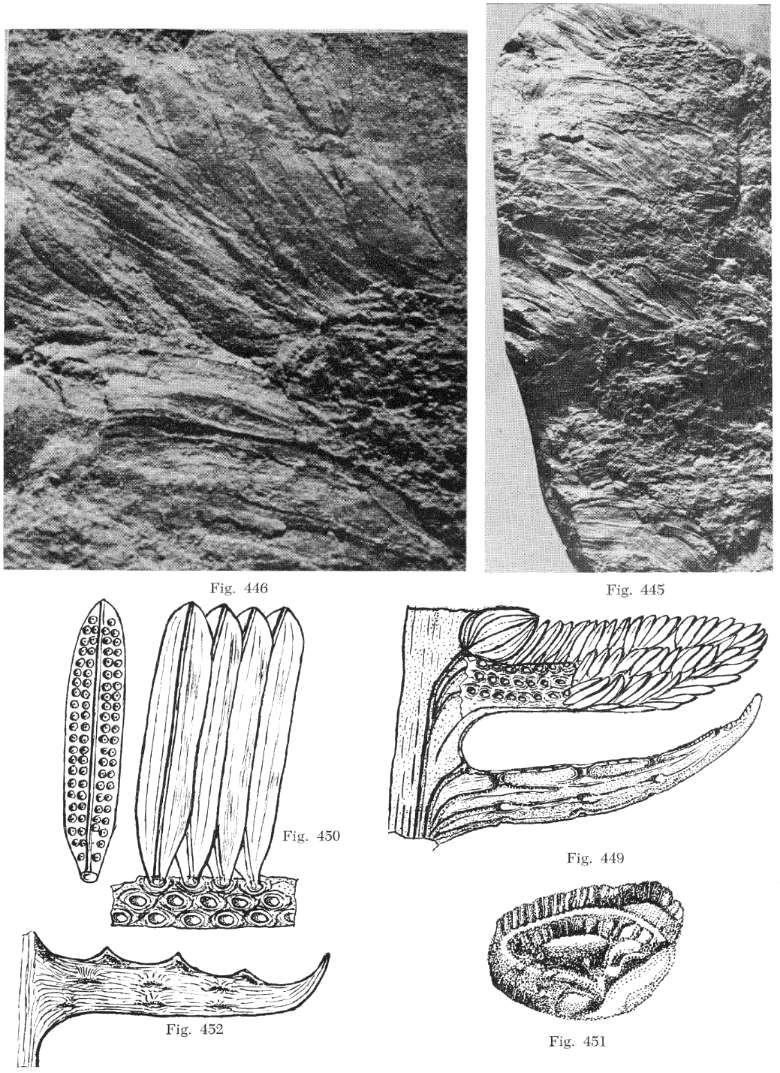
From Bock (1969: 269).
Discussion with Comparison
The name Primaraucaria
may conflict with some botanists' sensibilities, while giving others who have ulterior
motives a reason to reject it as a pre-Cretaceous angiosperm. There are many
examples in paleobotanical and palynological literature of incorrect names applied to new
fossils, because the author(s) made incorrect comparisons based on inadequate
information. Early Mesozoic polyplicate pollen possibly belonging to angiosperm
ancestors (i.e. stem group angiophytes) were originally thought to be the spores of
horsetails; the generic name given to these morphotypes was Equisetosporites.
That epithet must be used even though such palynomorphs are now known to be pollen
grains. The rules of botanical nomenclature were set up for such cases to conserve
those names in order to protect the discoverer(s) or author(s) of new species.
The description of fossils is
subject to more interpretation than descriptions of living organisms, which are complete
and undegraded. Being able to associate all preserved organs of fossil plants with
one taxon is sometimes difficult if not impossible without organic connection. In
the case of Primaraucaria there are enough specimens in organic connection to
allow a reasonable interpretation of this plant, partly because its morphology is so
unique in the fossil record. Hopefully, the evidence given here will be evaluated
based on its merits, and not on how well it fits with popular concepts of angiosperm
evolution.
For this comparison with the Balanophoraceae, two
possible interpretations for the female flowers are considered:
Naked unicarpellate flowers grouped together
around a secondary (paniculate) inflorescence axis (e.g. Lothophytum)
or bract axis, where bracts are distally expanded to form a peltate head (e.g. Lathrophytum and Ombrophytum).
Apetallous multicarpellate (apocarpous) flowers
with an elongate receptacle, which may or may not expand distally to form a peltate head;
flowers attached directly to main axis of inflorescence (spike).
In this treatment, the second interpretation is
adopted for the following reasons:
The flowers of most primitive extant angiosperms
are multicarpellate (e.g. Amborella, Nymphaeales), although the Chloranthaceae
contain unicarpellate gynoecia (Endress, 1987).
Primitive flowers can have a well-developed
perianth (e.g. Amborella), or have a reduced perianth (monoclamydous), or lack a
perianth (e.g. Chloranthus, Hedyosmum, Sarcandra, and the
aquatic fossil Archaefructus). It cannot be assumed that basal angiosperm
flowers did not have significant floral variation, given that the flowers of Amborella
and the Nymphaeaceae are so different.
Comparison Between Extant Balanophoraceae and
Fossil Taxa
| extant |
extant |
extant |
Triassic |
Triassic |
Triassic |
Langsdorffia
hypogaea |
Lathrophytum
peckoltii |
Lophophytum
mirabile |
Primaraucaria
wielandii |
Primaraucaria
sp 2 |
Triassiflorites
grandiflora |
inflorescence
unisexual |
inflorescence
bisexual,
female flowers located below male flowers |
inflorescence
bisexual,
female flowers located below male flowers |
inflorescence
unisexual |
inflorescence
bisexual,
female flowers located above male flowers |
inflorescence
bisexual,
female flowers located below male flowers |
female flowers unicarpellate,
carpels sessile,
no receptacle,
without perianth,
crowded to form dense head,
no bracts |
female flowers multicarpellate,
carpels sessile,
on long receptacle,
without perianth,
end of receptacle expanded - peltate,
no bracts |
female flowers
multicarpellate,
carpels sessile,
on long receptacle,
without perianth,
positioned in axils of primary bracts |
female flowers
unicarpellate,
carpels stalked,
no receptacle,
without perianth,
crowded to form
dense head,
no bracts |
female flowers multicarpellate,
carpels stalked,
on long receptacle,
without perianth,
end of receptacle not expanded,
no bracts |
female flowers
multicarpellate,
carpels sessile,
on long receptacle,
with perianth,
positioned in axils of primary bracts |
male flowers
monoclamydous,
2-3 bracts,
long pedicels,
inflorescence subtended by elongate bracts |
male flowers
without perianth,
short pedicels,
crowded to form
dense head |
male flowers
without perianth,
anthers elongate,
crowded on catkin-like secondary branch
cf. Hedyosmum |
male flowers
monoclamydous,
2-3? bracts,
short pedicels, inflorescence subtended by elongate bracts |
male flowers
without perianth,
laminar pedicels,
anthers latrorse and embedded in lamina |
male flowers
without perianth,
anthers elongate,
crowded on catkin-like secondary branch
cf. Hedyosmum |
scale-like
leaves
on lower female
inflorescence axis |
peltate bracts
on lower
inflorescence axis |
elongate
bracts
on lower
inflorescence axis |
scale-like
leaves
on lower female
inflorescence axis |
unknown |
elongate
bracts
on lower
inflorescence axis |
| wet tropical |
wet tropical |
wet tropical |
wet tropical |
wet tropical |
wet tropical |
Based on the above comparisons, taking into consideration
preservation and missing data, Primaraucaria and Triassiflorites can be
favorably compared to the Balanophoraceae. The existence of trisulcate and
pentasulcate aperture types within the Balanophoraceae (Watson and Dallwitz,1992-2000: website)
may be a genetic throwback (reversal) to ancestral Crinopolles trisulcate and pentasulcate
pollen found in the same strata as Primaraucaria and Triassiflorites.
Hickey and Doyle (1977: Fig. 65), in their monographic paper on
Early-Mid Cretaceous angiosperm leaf evolution, proposed a reduction in leaf size and
complexity between an ancestral gymospermous condition (e.g. pteridosperm or cycadopsid)
and secondary expansion at the beginning of dicot leaf evolution in the Cretaceous.
However, they proposed a hypothetical xeromorphic intermediate leaf-type during
that transformation. More recently the phylogenetic mapping of functional traits
reveals that the basal lineages, Amborella, Austrobaileyales, and some
Chloranthaceae, share ecological and physiological traits linked to shady, disturbed, and
possibly wet habitats (Feild et al., 2003a; Feild et al.,
2003b). All fossils of possible pre-Cretaceous angiosperms have been found in wet
sedimentary facies (habitats) ranging from the margins of freshwater (non-alkaline) lakes
and oxbow lakes to coal swamps (Tidwell et al.,
1977; Cornet and Traverse, 1975; Cornet,1986; 1989a; 1989b; 1993b; 1996; Cornet and Olsen,
1990; Cornet and Habit, 1992, Hochuli and Feist-Burkhardt, 2004, Kirkland et al.,
2002; and Sun et al., 1998; 2002), making it more likely that angiosperms
first evolved in a wet habitat rather than a dry one.
The ecology and physiology of Austrobaileya scandens, for
example, is different from earlier hypotheses that the earliest angiosperms were
early-successional xeric shrubs, disturbance-loving herbs characterized by high capacity
for photosynthesis and water transport, or were aquatic herbs (Feild et al.,
2003b). Instead, basal lineages possess functional characteristics commonly
associated with seed plants and ferns adapted to low light, including absence
of palisades mesophyll tissue, low leaf photosynthetic rates, and
possibly strong reliance on vegetative reproduction for survival (Feild et al.,
2003b), which could favor the evolution of parasitism. Holoparasites are considered
highly derived, genetically reduced members of angiosperms with normal genomes (Nickrent,
2002). They also represent an extreme end member in low light survival, having
lost the capability to photosynthesize, opting instead for deriving their energy from
other plants. At least eight families derived from core eudicots and two families
from pre-eudicots have semi- and holoparasite members: Lauraceae and Hydnoraceae
(magnoliids), Santalales (six families), Balanophoraceae, Cynomoriaceae, Krameriaceae,
Rafflesiales (four families), Lennoaceae, Cuscutaceae, and Orobanchaceae (Nickrent,
2002).
Although Nickrent (pers. comm., 2004) does not think an angiosperm
parasite, once it has lost the genetic ability to photosynthesize, could ever regain that
ability (without intergeneric or interfamily hybridization with a photosynthetic
relative: cf. Kellogg and Bennetzen, 2004), other non-parasitic members of the
same family or order could have many of the same reproductive characteristics as the
parasites. The existence of those plants or ancestors is implied by the existence of
a parasitic seed plant in a wet tropical rain forest. Because angiosperms have a
propensity for evolving parasitic members, even in near basal branches of their
phylogenetic tree, it should not be surprising that basal parasitic angiosperms could
exist. Because of their unique habit (i.e. root
parasites probably on cycadophytes, gingkophytes, seed ferns, and/or tree ferns, all
with giant fronds and leaves: Macrotaeniopteris, Eogingkoites, Taeniopteris,
Sphenobaera, Stangerites, etc., which make up the dominant vegetation in
sedimentary layers containing Primauraucaria - Cornet and Olsen, 1990; Axsmith et
al., 1995), wet habitat (i.e. tropical and subtropical rain forests
and swamps), and tropical distribution, their fossil record would be
sparce and limited to geologic facies that could preserve them in paleoequatorial regions,
such as those found in the Richmond
rift basin of Virginia.
Molecular data indicate that the Balanophoraceae do not represent
the phylogenetic root stock of angiosperms (Nickrent, 2002), but probably arose in the
nebulous group of angiosperms called "eudicots", which are thought to have first
appeared in the Early Cretaceous (Nickrent, pers. comm. 2004). It is more likely
that similarities between the Triassic fossils and some Balanophoraceae are due to
convergence rather than to familiar affinity. It is important to recognize that
verification of Sanmiguelia, Primaraucaria, and/or Triassiflorites
as crown-group (rather than stem group) angiosperms would not mean that the most
current phylogenetic trees based on taxonomic and molecular data are necessarily incorrect
(e.g. Fig. 7 in Doyle and Endress, 2000). It would instead mean that the basal stem
branches to the crown groups diverged prior to the Cretaceous and perhaps as early as the
Ladinian or Carnian (228-233 mya).
Wikström et al. (2001: molecular data) give a conservative
age for the origin of angiosperms as Middle Jurassic. Sanderson and Doyle (2001)
also give a Middle Jurassic age (180 mya ± 30 my) based on 18S rDNA data, but show that
for 1-2nd rbcL positions the age is basal Jurassic (200 mya ± 40 my). However,
Martin et al. (1992) give an estimated age of 300 mya ± 40 my based on cloned
and sequenced cDNAs. Wolfe et al. (1989: molecular data) give a maximum possible
age for the monocot-dicot divergence as early Late Triassic (230 mya), consistent with Sanmiguelia
(early Norian) in the Chinle Formation and the Crinopolles Group
of distinctive angiosperm-like pollen in the Carnian that combines monocot and dicot
pollen characteristics. If the discovery of a water-lily-like leaf (cf. Nuphar)
in basal Jurassic lacustrine strata of Utah is that of an angiosperm, the
age for origin would be no younger than Late Triassic. Molecular data has
consistently excluded (falsified) the Cretaceous as the time of angiosperm origin.
These estimates are based on an average mutation rate, but rapid evolution during
punctuated equilibria indicates that mutation rates may vary considerably depending on the
type of genetic alteration.
Conclusion
As more and more evidence for basal angiosperms has emerged in
molecular and paleobotanical fields, the origin of angiosperms has been pushed further
back in time from Early Cretaceous to Late Jurassic, then to Middle Jurassic, and now to
Early Jurassic, with indications that the age for angiosperm origin might ultimately reach
back to the Middle Triassic (Hochuli and
Feist-Burkhardt, 2004). Parasitic plants exist outside the Angiospermae, but they
are rare. For example, Cryptothallus
mirabilis is the only parasitic liverwort, while Parasitaxus
ustus (Podocaraceae) is the only parasitic conifer. Neither resembles Primaraucaria
or any member of the Balanophoraceae. Parasitaxus is found on New
Caledonia, the same island refuge of Amborella. Consequently, the presence
of a possible basal parasitic angiosperm in the Carnian (early Late Triassic) is not
surprising because 1) angiosperms are now thought to have initially evolved in dark
(understory) wet habitats rather than in bright dry ones, and 2) the taxonomic
distribution of parasitic angiosperms indicates a higher potential for parasitism in
angiosperms than in gymnosperms or cryptogams, with 10 parasitic families ranging from
pre-eudicots (magnoliids) to asterids (Nickrent,
2002). The existence of Primaraucaria implies that non-parasitic
relatives also must have existed then in or near the Richmond basin coal swamps.
This interpretation is supported by diverse multiaperturate angiosperm-like pollen found
in the same strata (Crinopolles Group: Cornet, 1989a).
References
Arber, A., 1961. Monocotyledons,
a morphological study. Reprinted by J. Cramer of Weinheim, Wheldon & Wesley, LTD.
and Hafner Publishing Co., New York, 258 p.
Axsmith, B.J., Taylor, T.N.,
Delevoryas, T., Hope, R.C., 1995. A new species of Eoginkgoites from the Upper
Triassic of North Carolina, USA. Rev. Paleobot. Palynol. 85(3):
189-198.
Bock, W, 1969. The American Triassic
Flora and Global Distribution. Geological Center, North Wales, Pennsylvania, Research
Series. v. 3 & 4: 406 p.
Cornet, B., 1977. The
palynostratigraphy and age of the Newark Supergroup. unpublished Ph.D. thesis, The
Pennsylvania State University, 505 p.
Cornet, B., 1986.
The reproductive structures and leaf venation of a Late Triasic angiosperm, Sanmiguelia
lewisii. Evol. Theory 7: 231-309.
Cornet, B., 1989a.
Late Triasic angiosperm-like pollen from the Richmond rift basin of Virginia, USA. Palaeontogr.
Abt. B 213: 37-87.
Cornet, B., 1989b.
The reproductive morphology and biology of Sanmiguelia lewisii, and its bearing
on angiosperm evolution in the Late Triassic. Evol. Trends in Plants 3:
25-51.
Cornet, B. and Olsen, P.E., 1990.
Early to middle Carnian (Triassic) flora and fauna of the Richmond and Taylorsville
basins, Virginia and Maryland, U.S.A. Guidebook No. 1, Virginia Museum of Natural
History, Martinsville, VA. 87 p.
Cornet, B., 1993a.
Applications and limitations of palynology in age, climatic, and paleoenvironmental
analyzes of Triassic sequences in North America. In Lucas, S.G. and Morales, M.
(eds.), The Nonmarine Triassic. New Mexico Museum of Natural History &
Science Bulletin No. 3: 75-83.
Cornet, B., 1993b.
Dicot-like leaf and flowers from the Late Triassic tropical Newark Supergroup rift zone,
U.S.A. Mod. Geol. 19: 81-99.
Cornet, B.,
1996. A new gnetophyte from the Late Carnian (Late Triassic) of Texas and its bearing
on the origin of the angiosperm carpel and stamen. In Taylor, D.W. and Hickey,
L.J. (eds.), Flowering Plant Origin, Evolution & Phylogeny. Chapter 3:
32-67. Chapman & Hall, New York.
Cornet, B. and Olsen, P.E., 1990.
Early to Middle Carnian (Triassic) flora and fauna of the Richmond and Taylorsville
basins, Virginia and Maryland, U.S.A. Virginia Museum of Natural History,
Guidebook No. 1, Martinsville, VA.
Cornet, B., and Habib,
D., 1992. Angosperm-like pollen from the ammonite-dated Oxfordian (Upper Jurassic) of
France. Rev. Palaeobot. Palynol. 71: 269-294.
Cornet, B., and
Traverse, A., 1975. Palynological contributions to the chronology and stratigraphy of
the Hartford basin in Connecticut and Massachusetts. Geoscience and Man, XI:
1-33.
Donoghue, M.J. and Doyle, J.A.,
2000. Seed plant phylogeny: Demise of the anthophyte hypothesis? Current Biology,
10: R106-R109.
Doyle, J.A., 2001. Significance of
molecular phylogenetic analyses for paleobotanical investigations on the origin of
angiosperms. Palaeobotanist 50: 167-188.
Doyle,
J.A. and Donoghue, M.J., 1993. Phylogenies and angiosperm diversification. Paleobiology
19(2): 141-167.
Doyle, J.A. and Endress, P.K., 2000.
Morphological phylogenetic analysis of basal angiosperms: comparison and combination with
molecular data. Int. J. Plant Sci. 161(6 Suppl.): 5121-5153.
Endress,
P.K., 1987. The Chloranthaceae: reproductive structures and phylogenetic position. Bot.
Jahrb. Syst., 109(2): 153-226.
Feild, T.S., Arens, N.C., and Dawson, T.E.,
2003a. The ancestral ecology of angiosperms: emerging perspectives from extant basal
lineages. Int. J. Plant Sci. 164(3 Suppl.):S129-S142.
Feild, T.S., Franks, P.J., and Sage, T.L., 2003b.
Ecophysiological shade adaptation in the basal angiosperm, Austrobaileya scandens
(Austrobaileyaceae). Int. J. Plant Sci. 164(2): 313-324.
Hickey,
L.J. and Doyle, J.A., 1977. Early Cretaceous fossil evidence for angiosperm evolution. Bot.
Rev. 43: 3-104.
Hochuli, P. and Feist-Burkhardt, S., 2004. A
boreal early cradle of Angiosperms? Angiosperm-like pollen from the Middle Triassic of the
Barents Sea (Norway). J. Micropalaeontol. 23(2): 97-104.
Kellogg, E.A. and Bennetzen, J.L., 2004. The
evolution of nuclear genome structure in seed plants. Amer. J. Bot. 91(10):
1709-1725.
Kent, D.V. and Olsen, P.E., 2000.
Implications of astronomical climate cycles to the chronology of the Triassic. Zbl.
Geol. Paläont. I: 1463-1473.
Kirkland, J.I., Lockley, M., and Milner, A.R., 2002. The St. George Dinosaur Tracksite. Survey
Notes, Utah Geological Survey, 34(3): 4-5. http://geology.utah.gov/surveynotes/snt34-3.pdf
Martin, W., Gieri, A.
and Saedler, H., 1989. Molecular evidence for pre-Cretaceous angiosperm origins. Nature
339(4 May): 46-48.
Martin, W., Lydiate, D., Brinkmann,
H., Forkmann, G., Saedler, H., and Cerff, R., 1993. Molecular Phylogenies in Angiosperm
Evolution. Mol. Biol. Evol. 10(1): 140-162.
Nickrent, D. L. 2002. Phylogenetic Origins of Parasitic Plants.
Chapter 3, pp. 29-56 In J. A. López-Sáez, P. Catalán and L. Sáez [eds.],
Parasitic Plants of the Iberian Peninsula and Balearic Islands. Mundi-Prensa, Madrid.
Olsen, P.E. and Kent, D.V., 2000.
High-resolution early Mesozoic Pangean climatic transect in lacustrine environments. Zbl.
Geol. Paläont. I: 1475-1495.
Olsen, P.E., Kent, D.V., Cornet, B.,
Witte, W.K., and Schlische, R.W., 1996. High-resolution stratigraphy of the Newark rift
basin (early Mesozoic, eastern North America. GSA Bulletin 108:
40-77.
Sanderson, M.J. and Doyle, J.A.,
2001. Sources of error and confidence intervals in estimating the age of angiosperms from rbcL
and 18S rDNA data. Amer. J. Bot. 88(8): 1499-1516.
Sun, G., Dilcher, D.L., Zheng, Z.,
and Zhou, Z., 1998. In Search of the First Flower: A Jurassic Angiosperm, Archaefructus,
from Northeast China. Science 282: 1692-1695.
Sun, G., Qiang, J., Dilcher, D.L.,
Zheng, S., Nixon, K.C., and Wang, X., 2002. Archaefructaceae, a new basal angiosperm
family. Science 296: 899-904.
Tidwell, W.D., Simper, A.D., and
Thayn, G.F., 1977. Additional information concerning the controversial Triassic plant: Sanmiguelia.
Palaeontogr. Abt. B 163: 143-151.
Winter, K.U., Becher, A., Münster,
T., Kim, J.T., Saedler, H., and Thiessen, G., 1999. MADS-box genes reveal that gnetophytes
are more closely related to conifers than to flowering plants. Proc. Natl. Acad. Sci.
USA 96: 7342-7347.
Wikström, N., V. Savolainen, and M. Chase, 2001. Evolution of the
angiosperms: calibrating the family tree. Proceedings of the Royal Society of London
Series B-Biological Sciences 268: 2211-2220.
Wolfe, K.H., M. Gouy, Y.-W. Yang, P.M. Sharp & W.-H.
Li., 1989. Date of the monocot-dicot divergence estimated from chloroplast DNA sequence
data. Proc. Natl. Acad. Sci. U.S.A. 86: 6201-6205.
Glossary
Monochlamydou
s
Referring to a flower that has only one perianth whorl (the calyx).
This page was created on 13 July 2003; it was last updated on 11/26/2014
© 2003 Bruce Cornet


















 For
comparison see an immature inflorescence of
For
comparison see an immature inflorescence of 









