Source link: Why.htm
Go back to page 1
The Stem and Root Anatomy of Sanmiguelia
lewisii,
and a Comparison with Extant Dicots and Monocots
by Bruce Cornet, Ph.D.
page 2
Table of
Contents
Page 1
Preparation for Discussion
Sanmiguelia Stem Anatomy
Growth
Habit
Habitat
and Adaptation
Comparison of Sanmiguelia
with Non-Arborescent Angiosperms
Herbaceous Rosettes (extant)
Herbaceous
Erects (extant)
Vines
and Lianas (extant)
Page 2
Comparison of Sanmiguelia
with Arborescent Monocots
What are the shared
characteristics?
Hypothetical
Transformation of Sanmiguelia
Burger's 1981
hypothesis that monocots came first
Discussion
Conclusions
References
Glossary
Source
publications
http://bcornet.tripod.com/evoltheo/Slewisii.htm
http://bcornet.tripod.com/evtrend/Sanmig2.htm
Comparison
of Sanmiguelia with Arborescent Monocots
 How would Sanmiguelia
have had to change its growth habit in order to become an arborescent dicot? If it
grew larger with more crown branching to support additional leaves, it might have
resembled Xanthorrhoea preissii or Yucca brevifolia (Agavaceae: Dahlgren
et al., 1985). It may have more closely resembled Dracaena marginata or D.
draco (Dragon tree, Dracaenaceae) with its multiple and upwards branching
stems. D. draco can reach many meters high and grow a trunk more than 2
meters thick (Dahlgren et al, 1985). Secondary growth in Dracaena is
similar to that of Dioscorea, and is not homologous with the secondary xylem of
dicots or of Sanmiguelia.
How would Sanmiguelia
have had to change its growth habit in order to become an arborescent dicot? If it
grew larger with more crown branching to support additional leaves, it might have
resembled Xanthorrhoea preissii or Yucca brevifolia (Agavaceae: Dahlgren
et al., 1985). It may have more closely resembled Dracaena marginata or D.
draco (Dragon tree, Dracaenaceae) with its multiple and upwards branching
stems. D. draco can reach many meters high and grow a trunk more than 2
meters thick (Dahlgren et al, 1985). Secondary growth in Dracaena is
similar to that of Dioscorea, and is not homologous with the secondary xylem of
dicots or of Sanmiguelia.
Dragon tree picture source: http://www.horticopia.com/hortpro.htm
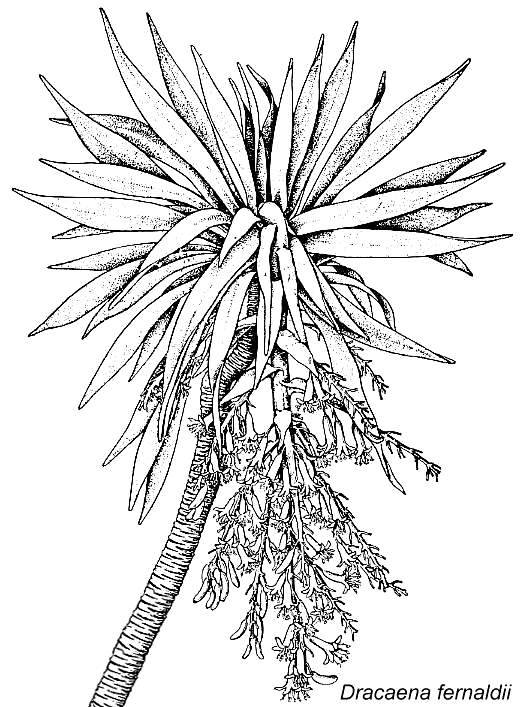
Modified from Dahlgren et al., 1985: Fig. 60.
Because its leaves were so large, it
could have resembled Musa (different leaf venation, however), but would not
have had a false axis or pseudostem like that of Musa and Veratrum.
Another difference is Sanmiguelia would not have had
well-developed petioles similar to those of Musa.
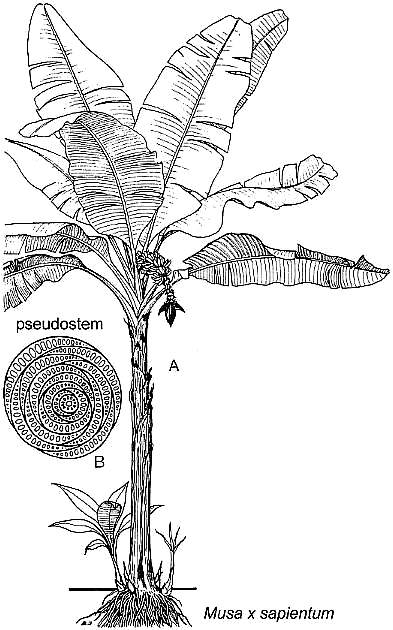
Modified from Dahlgren et al., 1985: Fig. 163.
The importance of Xanthorrhoea,
Yucca, and Dracaena is that they all re-evolved a secondary thickening
cambium which produces a secondary woody thickening comprised of crowded vascular bundles
separated by non-vascular tissue. Leaf traces have to pass through the equivalent of
wide (multiseriate) rays to innervate the leaves and clasping/sheathing leaf bases, like
that in Sanmiguelia. These taxa represent the only monocots that
significantly deviate from a more typical monocot habit. And yet they are far from
duplicating the diversity of arborescent and shrubby growth forms found in dicots.
In other words, even if Sanmiguelia had evolved a Dracaena type of
habit, that wouldn't have been enough to spark a phylogenetic radiation like that
documented in the Early Cretaceous (Lupia, 1999; Lupia et al., 1999; Lupia et al., 2000).
What are the
shared characteristics between Sanmiguelia, dicots, and monocots?
Table 1 from Burger (1981) with Sanmiguelia added. Color coded to show
intermediate characteristics.
| THE TYPICAL MONOCOT |
SANMIGUELIA |
THE TYPICAL DICOT |
| Small simple plants with few orders of
branching and little aerial axillary growth. |
Small simple plants with few orders of
branching and little aerial axillary growth. |
Larger complex plants with several orders of branching and much aerial
axillary growth. |
| Vascular bundles usually scattered, vasculature made up of
leaf traces in a loosely organized arrangement. Leaf gaps,
branch gaps and nodes poorly defined. |
Vascular bundles usually
becoming arranged in a highly organized vascular cylinder with internal cambium.
Leaf gaps, branch gaps and nodes poorly defined. |
Vascular bundles usually
becoming arranged in a highly organized vascular cylinder with internal cambium.
Leaf gaps, branch gaps and nodes clearly defined. |
| Secondary growth lacking or rarely developed and never with a
radial system. |
Secondary growth present, with
a well developed radial system. |
Secondary growth frequent,
almost always with a well developed radial system. |
| Prophyll solitary and lateral. |
Unknown - dicot condition implied. |
Prophylls usually paired and opposite. |
| Leaves almost always alternate,
not generally differentiated into petiole and lamina; leaf base usually merging without
differentiation into the stem. |
Leaves alternate, not generally
differentiated into petiole and lamina; leaf base usually merging without differentiation
into the stem. |
Leaves alternate, opposite or whorled, usually differentiated
into petiole and lamina; leaf base often clearly distinct from the tissues of the stem. |
| Laminae usually simple
and with few ranks of venation, reticulate tertiary veins
rare, margins usually entire,
trichomes generally rare. |
Laminae simple
and with four
clearly defined ranks of venation, reticulate
tertiary veins rare, margins entire,
trichomes common. |
Laminae simple or compound, usually with
several clearly defined ranks of venation,
tertiary venation usually reticulate, margins various, trichomes
common. |
| Embryo with a single cotyledon and a
lateral shoot apex; primary root fails to persist; unipolar growth. |
Embryo with opposing paired
cotyledons (one much smaller than the other), central
terminal shoot apex and a lateral shoot apex
(produced rhizome or stolon); primary root continues
growth; bipolar growth. |
Embryo with opposing paired
cotyledons and central terminal shoot apex;
primary root continues growth; bipolar growth. |
Hypothetical Transformation of Sanmiguelia
In order to understand what may have delayed an
angiosperm radiation until the Cretaceous, given that Sanmiguelia is a
Triassic dicot (Cornet, 1986; 1989a), what morphological and anatomical changes would have
to take place? An herbaceous origin of angiosperms is the current theory or paradigm
in botany (Hickey and Doyle, 1977; Doyle, 1977; Doyle and Donoghue, 1992; Doyle and
Endress, 2000). If the examples of herbaceous angiosperms listed in the tables above
are taken as the evolutionary end point or goal, the following changes in Sanmiguelia
vegetative and reproductive morphology and anatomy would have to take place.
Many times the obvious we don't see because it is
so familiar, and false assumptions and bias obscure our vision. Based on what we do
know about Sanmiguelia, its survival strategy was specialized or limited compared
to survival strategies we find today.
Changes Required for Sanmiguelia To Become an Extant
Herbaceous Monocot
Changes Required for Sanmiguelia To Become an Extant
Herbaceous Dicot
LEAF VENATION
Platanoid and nymphaeoid venation would precede pinnate venation.
 Modified from Hickey and Doyle, 1977: figs 26,
27, 43. |
Extant leaf comparison.
PETIOLE DEVELOPMENT
A leaf petiole would evolve as the sheathing leaf base was lost, and
leaf gaps would become more restricted in size and development. The multilacunar
node could persist (e.g. Rumex), but the number of
vascular traces to leaves would probably become reduced as a midrib or central vein became
better developed (Burger, 1981; cf. Hickey and Doyle, 1977). In this scenario,
trilacunar and unilacunar leaf gaps are derived (including the unilacunar leaf gap of Amborella).
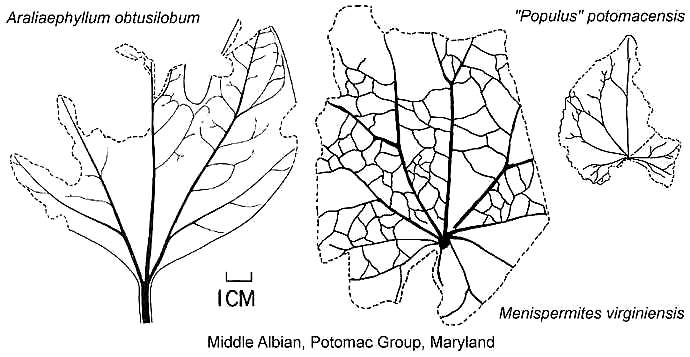
Burger (1981) shows an alternate hypothesis for the evolution of a
pinnately-veined leaf directly from a parallel-veined leaf without going through a
palmately-veined intermediate stage.
From Burger (1981).
The New Caledonia (Pacific island) shrub
with vesselless wood, Amborella
trichopoda, is considered to be the most primitive living angiosperm (USC; Harvard);
it has female flowers with the number of carpels ranging from five to eight. Its leaves
are alternate, simple, entire to pinnately lobed, and estipulate. Its ascidiate carpel is
not closed apically, and resembles the carpel of Sanmiguelia, which also was not
completely closed apically.


Picture source: http://www.ucsc.edu/currents/99-00/08-30/amborella.htm
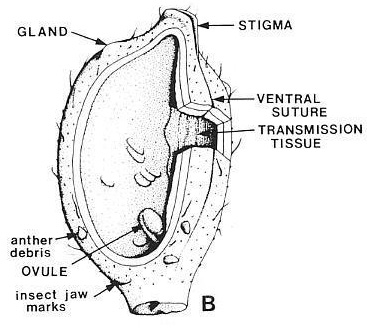
Modified from Cornet, 1989a. Interpretation of Sanmiguelia carpel
based on well-preserved fossils. Anther fragments and insect-jaw bite marks through
the carpel cuticle indicate that Sanmiguelia was insect pollinated. The
type of insect vector can only be surmised based on the hook-shaped jaw marks.
Beetles and the flying ancestors of ants and wasps had that type of mandible, and they
existed during the Late Triassic.
Therefore, comparing Sanmiguelia to a
primitive extant shrubby dicot will help show the amount of evolution needed before the
morphology and anatomy of Amborella trichopoda could be reached.
Changes Required for Sanmiguelia To Become an Extant
Shrubby Dicot
Discussion
From the discussion above, it should be clear by
now that the evolution of the dicot-type of leaf (pinnate venation, petiole, small leaf
gap, differentiation of leaf base from stem) was a major factor in the evolutionary
radiation of angiosperms in the Early Cretaceous. The argument that the closure of
the carpel was the stimulus for the Cretaceous radiation does not stand up to
scrutiny. The angiospermous reproductive strategy would give primitive angiosperms
an advantage over contemporaneous gymnosperms only if combined with other factors, such as
vegetative habit, pollination, and seed dispersal strategies.
"Flowers provide a record of
mode of pollination in addition to revealing a precise knowledge of taxonomic affinity.
In conjunction with a remarkably improved fossil record of insects (Grimaldi, D.A.,
1999), the history of floral form provides a more precise knowledge of the timing of
angiosperm-pollinator relationships and thus of angiosperm diversification vs. insect
diversification... And this relationship, essentially unique to angiosperms, has been
considered one of the foundations of relative angiosperm success... The pattern of
angiosperm radiation is consistent with the pattern of anthophilous insect radiation and
the pattern of appearance of derived floral characters and taxa specifically associated
with the most advanced anthophilous insects. There is a compelling similarity
between the rate of floral innovation/million years and the rate of angiosperm
diversification during the Cenomanian/Turonian interval coinciding with the first
occurrences of many derived insect pollinators." (Crepet, 2000).
Why is it that Amborella has survived with no other known
descendants or sister taxa if it was "superior" and separated from other dicots
at least as long ago as the beginning of the Cretaceous radiation (140 mya)? See
cladogram below. Having a closed carpel contributes little
to the most obvious (apparent) aspect of the Cretaceous radiation: leaf and vegetative
evolution. It is the evolution of the pinnately-veined leaf, the palmately-veined
leaf, and the pinnately-compound leaf in the Aptian and Albian that stands out in the
fossil record (along with implied growth habit and niche exploitation: e.g. Hickey and
Doyle, 1977). Pollen evolution (an aspect of floral evolution) lagged behind leaf
evolution in the Cretaceous (Lupia, 1999; Lupia et al., 1999), implying that reproductive
evolution was not necessarily the primary driving mechanism behind the Cretaceous
radiation. Floral evolution, however, may have been the quintessential speciation
mechanism, which limited the amount of leaf variation in any one species.
Crepet (2000) and
Grimaldi (1999) indicate (imply) that angiosperm success is not necessarily due to seeds
being protected inside an ovary (gymnosperms such as the Cheirolepidaceae, Araucariaceae,
and Ephedraceae effectively evolved an analogous condition), but rather due to a symbiotic
relationship with insects, where feedback through insect-driven selection determined
floral evolution, which accelerated pollinator evolution through Busselator-like
dynamics. The missing ingredient in the Cretaceous radiation was intelligent
selection, even though that intelligence may have been of the insect variety.
That ingredient (dedicated species-specific flower pollinators) did not exist prior to the
Cretaceous. Selection by humans has resulted in rapid diversification and
subspeciation/variation in horticultural plants, dogs, cats, bovines, horses, etc., which
to an outside observer might be mistaken for rapid evolution (i.e. radiation). Such
increases in apparent diversity are precursors to speciation.
By contrast, Sanmiguelia apparently
was incapable of sparking a similar radiation with its parallel-veined leaf, sheathing
leaf base, and simple, tectate-granular imperforate monosulcate pollen. How
do we know this?
Because it didn't!
Excluding Sanmiguelia from the history of
angiosperm evolution does not make the Cretaceous, angiosperm reproductive
superiority hypothesis correct by default. If the megafossil and
pollen discoveries in the Late Triassic are accepted as indications of angiosperm
evolution, there was a small basal radiation of primitive taxa
that may have set the stage for later evolutionary exploitation in the Cretaceous (see Why and cladogram below).
Composite cladogram showing the distribution of Sanmiguelia
characteristics plotted on Figure 7 from Doyle and Endress (2000). Other Triassic
data from Cornet (2003).
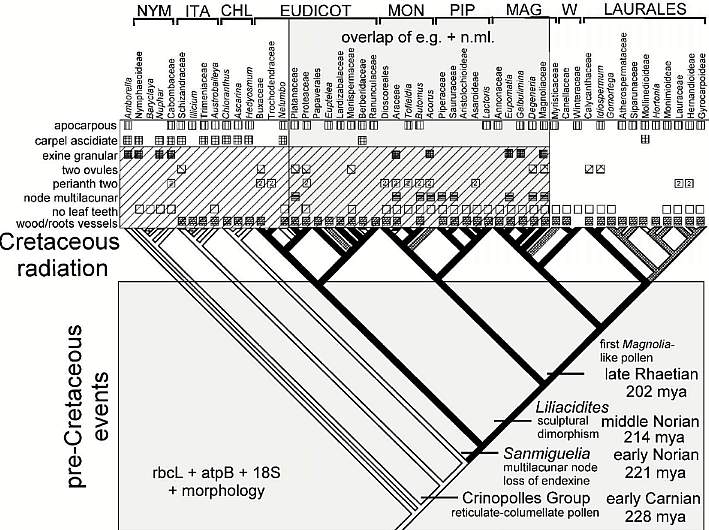
Modified
after Fig. 7, Doyle and Endress (2000). NYM = Nympaeales; ITA = named after Illicium,
Trimeniaceae, and Austrobaileya; CHL = Chloranthaceae; MON = Monocotyledonae; PIP
= Piperales; MAG = Magnoliales; W = Winterales.
Some critics of pre-Cretaceous evidence for
angiosperms ask why there are not more records of Jurassic angiosperms if they first
evolved during the Triassic. If one examines floral diversity and the number of new
families, orders, and classes of vascular plants in the Jurassic, and compares it to the
numbers found in the Triassic and Cretaceous, it will be noticed that the Jurassic was a
period of slow recovery after a mass extinction at the Triassic-Jurassic boundary.
Very little evolution above the family level occurred as floras in paleo-tropical and
subtropical regions of Pangea struggled to survive. Early and Middle Jurassic floras
are very similar at the genus and family level over large areas of the globe, being
dominated by relatively few taxa, most of which were adapted for aridity with small leaves
(microphyllous). The Cheirolepidaceae, Araucariaceae, Taxodiaceae, Podocarpaceae,
and Pinaceae dominated floras of central and southern Pangea (e.g. Australia and South
America) throughout the Jurassic, but not Eurasian floras of the northern hemisphere (see Jurassic floras
for diagrams and charts), which had more gymnosperms with large leaves.
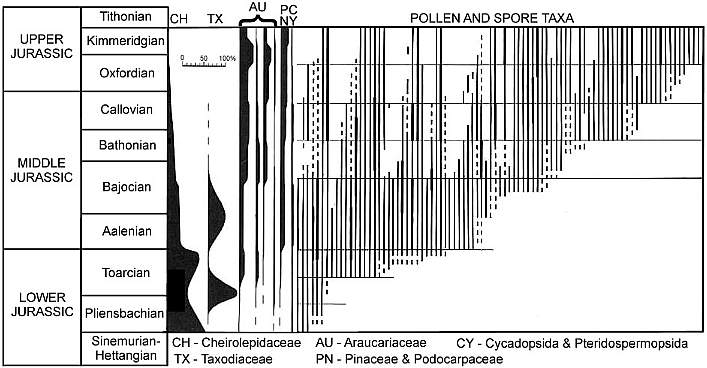
Modified from Filatoff (1975: Text-fig. 5); Jurassic palynology of the
Perth basin, western Australia.
Coal formation, an indication of wet climates, was
restricted to higher latitudes such as Scandinavia (Nilsson, 1958) and England (e.g.
Yorkshire delta: Couper, 1958) in the northern hemisphere (Laurasia) and Australia (de
Jersey, 1971; Filatoff, 1975) in the southern hemisphere (southern Gondwana). Coal
formation and wet climates in central Pangea shifted northward as Pangea drifted northward
(Olsen and Kent, 2000). In comparison, during the Late Triassic, coal formation
characterized basins in paleoequatorial regions, such as the Richmond basin of Virginia
(early to middle Carnian) and the Deep River basin of North Carolina (middle to late
Carnian: Cornet, 1993).
Following the mass extinction at the base of the
Jurassic, palynomorph composition and diversity increased slowly through the Jurassic as
climatic conditions improved in paleo-tropical and subtropical regions. As a
consequence, relatively few index palynomorphs evolved (i.e. common, widespread,
reliable), making it difficult to distinguish the different stages of the Jurassic using
only pollen and spores (Couper, 1958; Wall, 1965; de Jersey, 1971; Volkheimer, 1971; Volkheimer and Quattrocchio, 1975;
Filatoff, 1975; Vigran and Thusu, 1975; Cornet and Traverse, 1975; Thusu, 1978; Francis,
1983; de Jersey and Raine, 1990; Cornet and Habib, 1992; Cornet and McDonald, 2000).
Whatever the protracted cause for this evolutionary retardation appears to have affected
angiosperm survivors from the Triassic as much as it did pteridophytes, pteridosperms,
cycadeoids, and conifers. It wasn't until the Late Jurassic that we see floral
diversity improve to near the levels at the end of the Triassic. And it is also in
the Upper Jurassic (Oxfordian) that we occasionally find evidence of angiosperm pollen,
e.g. Clavatipollenites and Stellatopollis (Chloranthaceae and Liliales),
two important members of the Early Cretaceous radiation (Cornet and Habib, 1992).
The phylogenetic tree below
shows only those lineages that lead up to extant angiosperms (the survivors) and those
ancestral lineages we know about based on pollen and macrofossil evidence. The Steevesipollenites
pollen group (on the left), for example, which gave rise to the Crinopolles Group
of angiosperm-like pollen in the earliest Carnian, survived the mass extinction at the end
of the Triassic, and reappeared in the mid-Cretaceous where it underwent a second
palynological radiation (Elaterocolpites-Galeacornea complex: Stover,
1964; Jardiné,
1967). But that radiation of pollen morphotypes was restricted to the paleotropics
of western Africa, and those taxa became extinct at the end of the Cenomanian. It
was from this same paleogeographic area that Cretaceous angiosperms began their northward
and southward migration in the Aptian-Albian ((Doyle et al., 1977; Doyle, 1999).
Following a very wet climatic period in the
paleotropical regions of Pangea during the early Carnian, the climate gradually became
more arid, although it did so in a cyclical fashion according to Milankovitch
orbitally-forced cycles (Olsen and Kent, 2000).
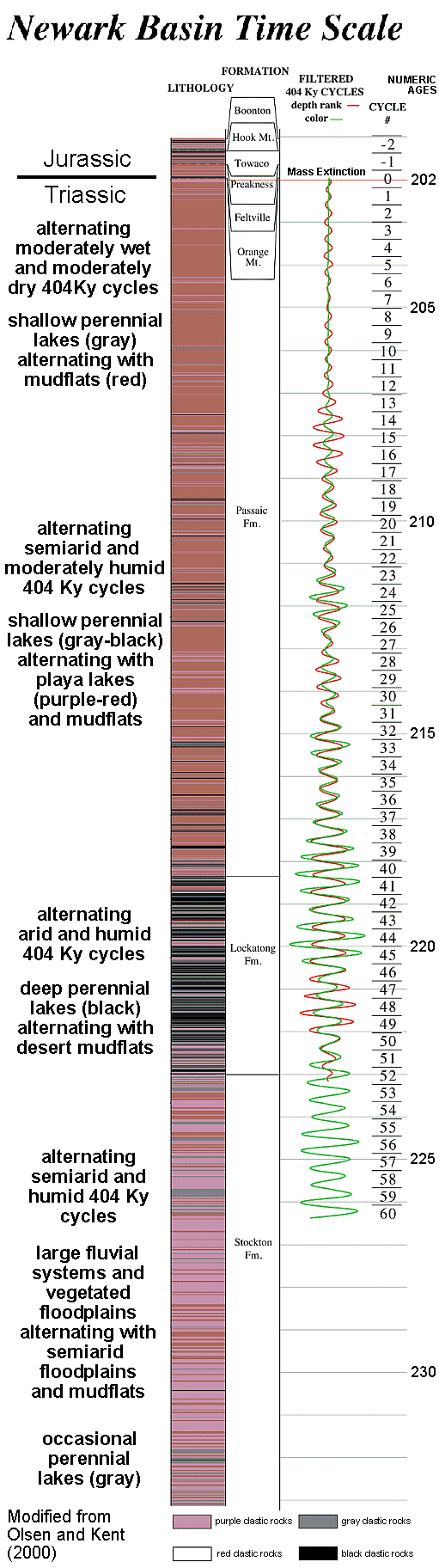
Within the wetter climatic cycles embedded within arid and semiarid
redbed facies of the Newark Supergroup, North America, angiosperm-like pollen appeared
again and again in small percentages (typically <1%). But with each successive
appearance of angiosperm-like pollen, new morphotypes were present. For example,
just prior to the mass extinction at the end of the Triassic Magnolia-like pollen
(cf. Magnoliales) and zonasulculate Monsteria-like (cf. Arales) or Schrankipollis-like
(cf. Winterales) pollen appeared. Winteroid pollen is quite diverse in the
Barremian-early Aptian of Gabon (Doyle, et al., 1990), implying a deeply-rooted ancestry.

SEM and TEM examples of latest Rhaetian magnoliid angiosperm-like pollen
(Cornet, unpublished study).
Postulated angiosperm
phylogenetic tree based on most recent taxonomic and molecular data.
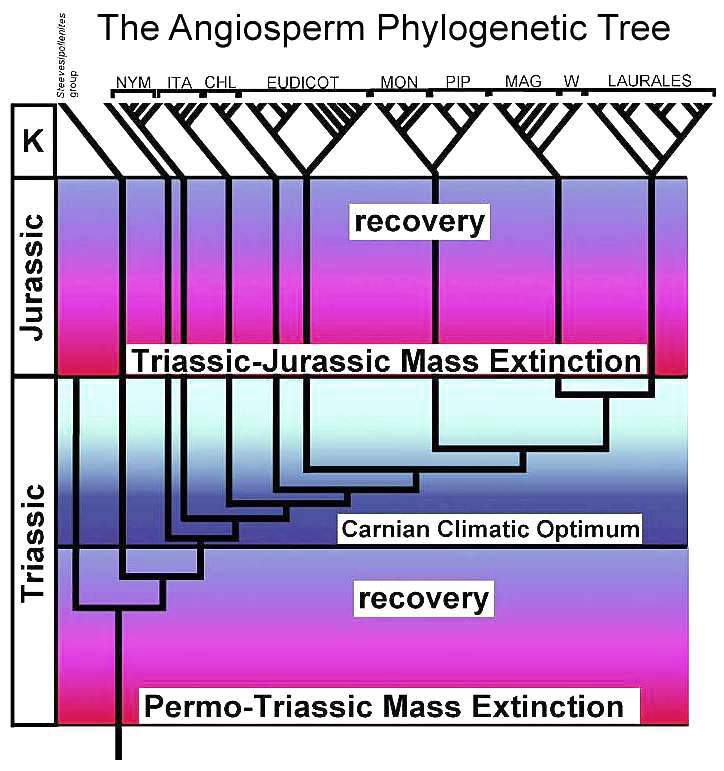
NYM = Nympaeales; ITA = named after Illicium,
Trimeniaceae, and Austrobaileya; CHL = Chloranthaceae; MON = Monocotyledonae; PIP
= Piperales; MAG = Magnoliales; W = Winterales.
Major climatic shifts coordinated with continental
drift, the opening of the Atlantic Ocean, and a global mass extinction event certainly
played a significant role in the delayed timing of a sustained angiosperm radiation.
In addition, long-leaved, rapid-growing, flower-producing beetle-pollinated cheirolepid
conifers evolved in the Early Jurassic of central Pangea (Hartford basin: Cornet
and McDonald, 2000). Their flowers resembled those of asters, while their
immature fruits resembled Magnolia fruits. They had ovules contained within
a closed ovary with stigmatic pollen germination. They may have been formidable
competitors with primitive Jurassic angiosperms (an analogous situation existed between
dinosaurs and early mammals, both in size and dominance). These conifers lived in
the same riparean habitats that angiosperms occupied during the Early Cretaceous (Cornet
and McDonald, 2000). We don't see angiosperms dominating those habitats
until cheirolepid conifers became extinct in the Cenomanian (Lupia, 1999; Lupia et al.,
1999). Their flowers/fruits may even have been mistaken for angiosperm flowers (e.g.
Lesqueria: Crane and Dilcher, 1984; Cornet, 2002).
Conclusions
Burger (1981) details six hypothetical trends in
the early evolution of angiosperms:
Small simple plants preceded large complex plants in the early
evolution of angiosperms.
Scattered vascular bundles within the stem preceded a tubular
vasculature with included cylindrical cambium.
Simple leafy stems without aerial branching preceeded complex woody
growth with several orders of branching.
Simple undifferentiated leaves preceded leaves with a clearly
differentiated petiole and lamina.
Leaves with one or a few poorly differentiated orders (ranks) of
venation preceded leaves with several clearly differentiated orders of venation.
A clasping leaf base continuous with the tissue of the stem
preceded a leaf base clearly differentiated from the stem; deciduous leaves evolved later
and were a major innovation.
Does Sanmiguelia adequately test Burger's
monocot origin hypothesis? If one just takes a look at extant monocots, one will
find verification of all the above trends, even though secondary growth from a cylindrical
cambium does not produce a dicot-type of secondary wood. The dicot-type of leaf has
evolved in several different lineages. Monocot vines have evolved which compare
uncannily with dicot vines (e.g. Dioscorea spp. and Smilax spp.; see
Vines and Lianas table above), including
deciduous leaves with petioles, several orders of venation, palmate venation, and cross
veins with closed loops. Smilax rotundifolia (common greenbrier) even has thorns,
more characteristic of dicot vines and shrubs. If monocots could do it once, how
about twice?
If all dicots on Earth today were to disappear,
how many vacated niches would the monocots and gymnosperms be able to occupy? How
long would it take for monocots to undergo a radiation, and replace the various vegetative
and reproductive patterns we see in dicots? These are questions that need to be
considered when considering pre-Cretaceous angiosperm evolution.
Monocots produce some of the most primitive types
of pollen of any angiosperm group based on the fossil record. Polyplicate pollen is
common among some tribes of Araceae, and has been shown to be the precursor pollen type to
the evolution of tectate-reticulate-columellate monosulcate pollen in the Late Triassic
(see Cornet, 1989b
and Why do Paleobotanists Believe
in a Cretaceous Origin of Angiosperms?).
Even some recent
molecular data indicates that the monocot Oryza (i.e. grasses) is the sister
taxon to all other angiosperms (Sanderson and Doyle, 2001; see their Figure 2 below),
while the Araceae come next, then the Palmae. This tree also shows the lineages to Cycas,
Zamia, and Ginkgo separating later than the fossil record
suggests. Whether these possibilities can be adequately tested and proven or
falsified with more data remains to be seen, but what it does do is demonstrate that there
are plausible alternative theories for the origin of angiosperms besides the popular
paradigm, which excludes Sanmiguelia because of its monocot origin implications.
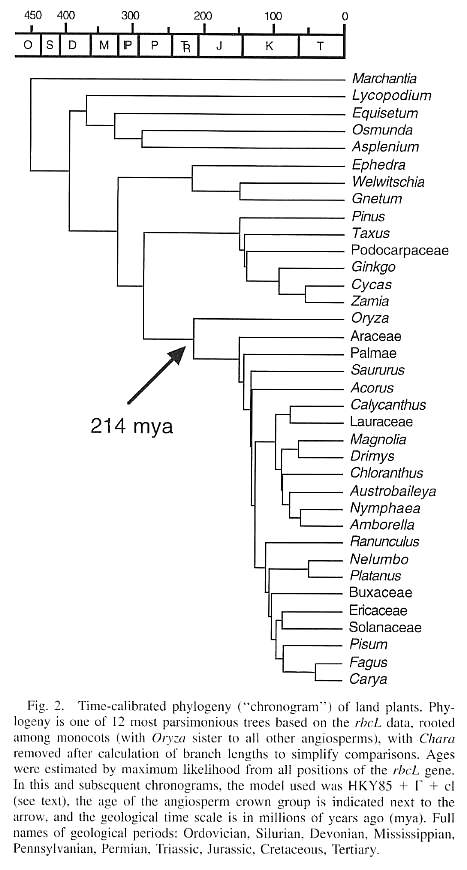
From Sanderson and Doyle, 2001.
Thus the question
boils down to this: Does Sanmiguelia represent an early stage of dicot evolution
from a monocot ancestor, or does Sanmiguelia represent an early stage of monocot
evolution from a dicot ancestor? Or could there be a third solution? Did the
ancestors of extant angiosperms occupy an intermediate position which was neither monocot
or dicot, sensu stricto? Could Sanmiguelia be an
indication of orders and subclasses of pre-Cretaceous angiosperms that are now
extinct? Are botanists trying to solve a puzzle where important pieces are still
missing, and concepts of basal angiosperms generated without those data are incomplete or
even misleading? Might the ancestors of dicots and monocots belong to neither
subclass? For example, consider the parasite family, Balanophoraceae,
which has an extensive pantropic
distribution, implying an origin before the supercontinental breakup of Pangea (i.e.
pre-Cretaceous), and a basal phylogenetic position indicated by molecular data?
References
Arber, A., 1961. Monocotyledons,
a morphological study. Reprinted by J. Cramer of Weinheim, Wheldon & Wesley, LTD.
and Hafner Publishing Co., New York, 258 p.
Arber, A., 1972. Water Plants, a
study of aquatic angiosperms. Reprinted by V. Von J. Cramer of Lehre, Wheldon &
Wesley, LTD., Stechert-Hafner Services Agency, Inc., New York, 436 p.
Burger, W.C., 1981. Heresy revived:
The monocot theory of angiosperm origin. Evolutionary Theory 5:
189-223.
Cornet, B., 1986.
The reproductive structures and leaf venation of a Late Triasic angiosperm, Sanmiguelia
lewisii. Evol. Theory 7: 231-309.
Cornet, B., 1989a.
The reproductive morphology and biology of Sanmiguelia lewisii, and its bearing
on angiosperm evolution in the Late Triassic. Evol. Trends in Plants 3:
25-51.
Cornet, B., 1989b.
Late Triasic angiosperm-like pollen from the Richmond rift basin of Virginia, USA. Palaeontogr.
Abt. B 213: 37-87.
Cornet, B., and Habib,
D., 1992. Angosperm-like pollen from the ammonite-dated Oxfordian (Upper Jurassic) of
France. Rev. Palaeobot. Palynol. 71: 269-294.
Cornet, B., and McDonald, N.G.,
2000. A new cheirolepidaceous conifer bearing flowers from the Early Jurassic of
Connecticut, USA. Unpublished manuscript at http://bcornet.homestead.com/files/Conanthus/conanthus.htm
Cornet, B. and Traverse, A., 1975. Palynological contributions to the
chronology and stragigraphy of the Hartford basin in Connecticut and Massachusetts. Geoscience
and Man, 11: 1-33.
Couper, R.A., 1958. British Mesozoic microspores and pollen grains, a
systematic and stratigraphic study. Palaeontographica B, 103:
75-179.
Crane, P.R. and Dilcher, D.L., 1984.
Lesqueria: An early angiosperm fruiting axis from the mid-Cretaceous. Ann.
Missouri Bot. Gard. 71: 384-402.
Crepet, W.L., 2000. Progress in
understanding angiosperm history, success, and relationships: Darwin's abominably
"perplexing phenomenon". Proc. Natl. Acad. Sci. USA, Commentary,
97(24): 12939-12941.
Dahlgren, R.M.T., Clifford, H.T.,
and Yeo, P.F., 1985. The Families of the Monocotyledons. Springer-Verlag, New
York, 520 pp.
de Jersey, N.J., 1971. Early
Jurassic miospores from the Helidon Sandstone. Geological Survey of Queensland,
Pub. 351, Palaeontological Papers: 25: 1-49.
de Jersey, N.J. and Raine, J.I., 1990. Triassic and earliest Jurassic
miospores from the Murihiku Supergroup, New Zealand. New Zealand Geol. Surv. Paleont.
Bull. 62, 164 pp.
Doyle, J.A., 1977. Patterns of
Evolution in Early Angiosperms. In Hallam, A. (ed.), Patterns of Evolution. Elsevier
Scientific Publishing Company, Amsterdam. Chapter 16: 501-546.
Doyle, J.A., 1999. The rise of
angiosperms as seen in the African Cretaceous pollen record. In Heine, K. (ed.),
Palaeoecology of Africa and the surrounding islands. Proceedings of the Third Conference
on African Palynology, Johannesburg, 14-19 September 1997. A.A.Balkema,
Rotterdam, Brookfield: 3-29.
Doyle, J.A., Biens, P., Doerenkamp,
A., and Jardiné, S., 1977. Angiosperm pollen from the pre-Albian Lower Cretaceous of
equatorial Africa. Bull. Cent. Rech. Explor.-Prod. Elf-Aquitaine 1(2):
451-473.
Doyle, J.A. and Donoghue, M.J.,
1993. Phylogenies and angiosperm diversification. Paleobiology 19(2):
141-167.
Doyle, J.A. and Endress, P.K., 2000.
Morphological phylogenetic analysis of basal angiosperms: comparison and combination with
molecular data. Int. J. Plant Sci. 161(6 Suppl.): 5121-5153.
Doyle, J.A., Hotton, C.L., and Ward,
J.V., 1990. Early Cretaceous tetrads, zonasulculate pollen, and Winteraceae. I. Taxonomy,
morphology, and ultrastructure. Amer. J. Bot. 77(12): 1544-1557.
Endress, P.K., 1987. The
Chloranthaceae: reproductive structures and phylogenetic position. Bot. Jahrb. Syst.,
109(2): 153-226.
Esau, K., 1966. Anatomy of Seed
Plants. John Wiley and Sons, Inc., New York, 376 p.
Fernald, M.L., 1970. Gray's
Manual of Botany. D.Van Nostrand Company, New York, 1632 pp.
Filatoff, J., 1975. Jurassic palynology of the Perth basin, western
Australia. Palaeontographica B, 154: 1-113.
Francis, J.E., 1983. The dominant conifer of the Jurassic Purbeck
Formation, England. Palaeont., 26: 277-294.
Grimaldi, D.A., 1999. The co-radiations
of pollinating insects and angiosperms in the Cretaceous. Annals of the
Missouri Botanical Garden, 86, 373-406.
Groff, P.A. and Kaplan, D.R., 1988. The relation of root systems to
shoot systems in vascular plants. Bot. Rev. 54(4): 387-422.
Hickey, L.J. and Doyle, J.A., 1977.
Early Cretaceous fossil evidence for angiosperm evolution. Bot. Rev. 43:
3-104.
Jardiné, S., 1967.
Spores a expansions en forme d'elateres du Cretace Moyen d'Afrique occidentale. Rev.
Palaeobot. Palynol., 1: 235-58.
Lupia, R., 1999. Discordant
morphological disparity and taxonomic diversity during the Cretaceous angiosperm
radiation: North American pollen record. Paleobiology 25(1):
1-28.
Lupia, R., Lidgard, S., and Crane,
P.R., 1999. Comparing palynological abundance and diversity: implications for biotic
replacement during the Cretaceous angiosperm radiation. Paleobiology 25(1):
305-340.
Lupia, R., Crane, P.R., and Lidgard,
S., 2000. Angiosperm diversification and Cretaceous environmental change. In
Culver, S.J. and Rawson, P.F. (eds.), Biotic Response to Global Change, The Last 145
Million Years 15: 207-222. Cambridge University Press, U.K.
Newcomb, L., 1977. Wildflower
Guide. Little, Brown and Company, New York, 490 pp.
Nilsson, T., 1958. Uber das
Vorkommen eines mesozoischen Sapropelgesteins in Schonen. Lunds Universitets Arsskrift,
54(2): 111 p.
Cornet, B., 1993. Applications and
limitations of palynology in age, climatic, and paleoenvironmental analyzes of Triassic
sequences in North America. Lucas, S.G. and M. Morales, eds., 1993. The Nonmarine
Triassic. New Mexico Museum Of Natural History & Science Bulletin No.3, p. 75-93.
Sanderson, M.J. and Doyle, J.A.,
2001. Sources of error and confidence intervals in estimating the age of angiosperms from rbcL
and 18S rDNA data. Amer. J. Bot. 88(8): 1499-1516.
Stover, L.E., 1964.
Cretaceous ephedroid pollen from West Africa. Micropaleont., 10:
145-156.
Taylor, Thomas N. and Taylor, Edith
L., 1993. The Biology and Evolution of Fossil Plants. Prentice-Hall, Inc., A
Simon & Schuster Company, Englewood Cliffs, New Jersey, 982 p.
Thusu, B., 1978. Distribution of
biostratigraphically diagnostic dinoflagellate cysts and miospores from the northwest
European continental shelf and adjacent areas. IKU (Continental Shelf Institute),
Trodheim, Norway, Pub. 100, 111 p.
Tidwell, W.D., Simper, A.D., and
Thayn, G.F., 1977. Additional information concerning the controversial Triassic plant: Sanmiguelia.
Palaeontogr. Abt. B 163: 143-151.
Vigran, J.O. and Thusu, B., 1975.
Illustrations and distribution of the Jurassic palynomorphs of Norway. Royal Norwegian
Council for Scientific and Industrial Research (NTNF) Continental Shelf Division,
Pub. 65, 55 p.
Volkheimer, W., 1971. Algunos
adelantos en la microbioestratigrafia del Jurasico en la Argentina y comparacion con otras
regiones del hemisferio austral. De Ameghinana, 8: 341-355.
Volkheimer, W. and Quattrocchio, M.,
1975. Sobre el hallazgo de microfloras en el Jurasico Superior del borde austral del la
Cuenca Neuquina (Republica Argentina). Actas I, Congreso Argentino de Paleontogia y
Bioestratigraphia, 1: 589-615.
Wall, D., 1965. Microplankton, pollen, and spores from the Lower
Jurassic in Britain. Micropaleontology, 11: 151-190.
Glossary
amphivasal:
amphi for around or on both sides; vas L. for vessel or vasculature.
angiosperm:
Any plant of the class (Angiospermae) having the seeds in an enclosed ovary.
arborescent:
Resembling a tree in growth structure or appearance; tree-like.
bulb: A
bud, usually underground, consisting of a short, thick stem sending out roots from below,
and beqring overlapping, scalelike leaves, as in the lily, onion, or tulip; a fleshy tuber
or corm resembling a bulb, as in a dahlia bulb.
bundles: A
general reference to vascular tissue organized into discrete circular to elliptical or
lunate-shaped (cross section) clusters of fluid-conducting cells (xylem and phloem) that
pass like "rods" vertically through the stem.
cambium: A
meristem with products of division arranged orderly in parallel files. Preferably applied
only to the two lateral meristems, the vascular cambium and the cork cambium
or phellogen. Consists of one layer of initials and their undifferentiated
products, or derivatives.
corm: A
short, bulblike, underground, upright stem, invested with a few thin membranes or scale
leaves, as in the crocus or gladiolus.
cutin: A
wax-like highly complex fatty substance present in plants as an impregnation of epidermal
walls and as a separate layer, the cuticle, on the outer surface of the epidermis. Makes
the walls more or less impervious to water.
dioecious:
A condition in which ovule- and pollen-bearing structures are borne on separate plants;
all dioecious plants have unisexual reproductive structures (contrast
monoecious).
dicotyledonous
(dicot): An angiosperm embryo having two cotyledons (first leaves); a member of the
dicots.
disulcate:
A pollen type having two (normally distal) sulci.
ephemeral:
Short-lived, transient.
eudicot:
First or earliest record of higher extant dicots.
floodplain:
The flat land on either side of a river or stream which has been shaped by water erosion
and sedimentary deposition.
habit: The
form a plant takes, such as a tree, shrub, erect herb, rosette herb, aquatic herb, etc.
herbaceous:
A plant having little or no secondary development; nonwoody.
interfascicular
cambium: Vascular cambium giving rise to secondary xylem and interfascicular rays.
intrafascicular
cambium: Vascular cambium restricted to a vascular bundle, or fascicle; not
connected with cambia of other vascular bundles.
leaf gap:
In the nodal region of a stem. A region of parenchyma in the vascular cylinder occurring
where a leaf trace is bent away from the vascular system of the stem toward the leaf.
leaf trace:
A vascular bundle in the stem extending between its connection with a leaf and that with
another vascular unit in the stem. A leaf may have none or more traces. Sometimes the
whole complex of traces of one leaf is called a leaf trace.
meristem:
Tissue primarily concerned with protoplasmic synthesis and formation of new cells by
division - responsible for stem, root, and leaf growth.
microphyllous:
A plant having small bract-shaped leaves characteristic of arid and semiarid habitats.
monocotyledonous
(monocot): An angiosperm embryo having only one cotyledon (first leaf); a member of
the monocots.
monoecious:
Bearing both pollen- and ovule-producing organs on the same plant; the organs can be
either unisexual or they can be bisexual (both types in a single fructification )(contrast
dioecious).
monopodial:
Stem or main axis arising from a terminal meristem.
multilacunar
node: multi L. for many, more; lacuna L.
for cavity, hollow, cavern; in reference to the number of spaces or gaps in a vascular
cylinder for leaf and branch vascular traces.
niche: A
unique environmental area that provides all of the essential physical and chemical
elements for a plant or animal to survive, grow and reproduce.
node (nodal):
That part of the stem at which one or more leaves are attached. Not clearly delimited
anatomically.
palmate:
Branching outwards from a common point, like fingers on a hand.
periderm:
Secondary protective tissue derived from the phellogen (cork cambium) and replacing the
epidermis, typically in stems and roots. Consists of phellem (cork), phellogen, and
phelloderm.
petiole:
The slender stem that supports the blade of a foliage leaf; a leafstalk.
phellem (cork):
Protective tissue composed of nonliving cells with suberized walls. Replaces the epidermis
in one-year and older stems and roots of many plants and formed by the phellogen (cork
cambium). Part of the periderm.
phelloderm:
A tissue formed by the phellogen in the opposite direction of the cork. Resembles cortical
parenchyma. Part of the periderm.
phellogen (cork
cambium): A lateral meristem forming the periderm, a protective tissue common in
stems and roots of dicotyledons and gymnosperms. Produces phellem (cork) toward the
surface of the plant, phelloderm toward the inside.
phloem: The
principal food-conducting tissue of the vascular plant, basically composed of sieve
elements, parenchyma cells, fibers, and sclereids.
phylogeny:
The race history of an animal or plant type (contrast ontogeny).
phyllotaxy:
The arrangement of leaves on a stem.
pinnate:
Branching at right angles as in a fern frond.
procambium:
Primary meristem or meristematic tissue which differentiates into the primary vascular
tissue. Also called provascular tissue.
primary xylem:
Xylem tissue differentiating from procambium during primary growth and differentiation of
a vascular plant. Commonly divided into the earliest protoxylem and the later metaxylem.
Not differentiated into axial and ray systems.
protoxylem:
The first formed elements of the xylem in a plant organ. First part of the primary xylem.
radiation:
An evolutionary/genetic process by which diversity rapidly increases and new species,
genera, and families of animals or plants evolve from one or more closely closely-related
ancestors; if the radiation continues long enough (millions of years), new orders,
subclasses, and classes of animals and plants can evolve.
rhizome:
horizontal stem, whether lying on the ground (prostrate) or growing below ground.
riparean:
stream banks.
scalariform
pitting: Elongate pits arranged parallel so s to form a ladder-like (scalariform)
pattern.
selfing: The reproductive process by which a plant fertilizes itself.
sessile:
Attached directly by the base; not raised upon a stalk or petiole.
sheathing base:
Applied to the base of a leaf, either sessile or petiolate, when the leaf base encircles
the stem.
stelar:
Relating to or of the stele.
stele:
conceived by Van Tieghem as a morphological unit of the plant body comprising the vascular
system and the associated ground tissue (pericycle, inerfascicular regions, and pith). The
central cylinder of the axis (stem and root).
stolon: a
runner or rootstock used to propogate certain grasses.
suberin:
The same definition as for cutin with which it is closely related.
suberization
(suberized): Impregnation of the wall with suberin or deposition of suberin lamellae in
the wall.
sympodial:
The apparent main stem does not develop from a terminal bud, but is made up of successive
secondary axes, each of which represented one branch of a dichotomy, the other branch
being a weaker growth that gives rise to specialized axes such as inflorescences.
tracheary element:
General term for a water-conducting cell, tracheid or vessel member.
trilacunar node: tri L. for three; lacuna L. for cavity,
hollow, cavern; in reference to the number of spaces or gaps in a vascular cylinder for
leaf and branch vascular traces.
tuber: A
potato-shaped swelling of a root, which serves as a storage organ for nutrients.
unilacunar node: uni L. for one; lacuna L. for cavity,
hollow, cavern; in reference to the number of spaces or gaps in a vascular cylinder for
leaf and branch vascular traces.
vascular cylinder:
A term of convenience applied to the vascular tissue and associated ground tissue in stem
or root. Refers to the same part of stem or root that is designated stele but
without the theoretical implications of the stelar concept. Same as central cylinder.
vascular:
Possessing specialized water-conducting cellular elements.
vascular trace:
water- and food-conducting tissue and associated ground tissue passing from the stem to a
leaf or branch.
xylem: The
principal water-conducting tissue in vascular plants characterized by the presence of
tracheary elements. The xylem may be also a supporting tissue, especially the secondary
sylem (wood).
The glossary was created with the help
of glossaries and definitions in the following books:
Anatomy of Seed Plants by Katherine Esau (1966)
Composition of Scientific Words by Roland W. Brown (1978)
Webster's New Collegiate Dictionary (1961)
Return
to page 1
Return to Why
This web page was created on 6 March
2003, and was last modified on 11/10/2005.

 How would Sanmiguelia
have had to change its growth habit in order to become an arborescent dicot? If it
grew larger with more crown branching to support additional leaves, it might have
resembled Xanthorrhoea preissii or Yucca brevifolia (Agavaceae: Dahlgren
et al., 1985). It may have more closely resembled Dracaena marginata or D.
draco (Dragon tree, Dracaenaceae) with its multiple and upwards branching
stems. D. draco can reach many meters high and grow a trunk more than 2
meters thick (Dahlgren et al, 1985). Secondary growth in Dracaena is
similar to that of Dioscorea, and is not homologous with the secondary xylem of
dicots or of Sanmiguelia.
How would Sanmiguelia
have had to change its growth habit in order to become an arborescent dicot? If it
grew larger with more crown branching to support additional leaves, it might have
resembled Xanthorrhoea preissii or Yucca brevifolia (Agavaceae: Dahlgren
et al., 1985). It may have more closely resembled Dracaena marginata or D.
draco (Dragon tree, Dracaenaceae) with its multiple and upwards branching
stems. D. draco can reach many meters high and grow a trunk more than 2
meters thick (Dahlgren et al, 1985). Secondary growth in Dracaena is
similar to that of Dioscorea, and is not homologous with the secondary xylem of
dicots or of Sanmiguelia. 









