Primary Reference
|
Palaeontographica
Abt. B |
213 |
Lfg. 1-3 |
37-87 |
Stuttgart,
August 1989 |
LATE TRIASSIC ANGIOSPERM-LIKE
POLLEN
FROM THE RICHMOND RIFT BASIN OF
VIRGINIA, U.S.A.
BY
BRUCE CORNET*)
With 9 Plates and
12 Text-figures in the Text
Summary
The Richmond Basin
is a small intracratonic rift basin containing some of the oldest strata in the
Newark Supergroup of eastern North America. Recent drilling has established for
the first time an accurate picture of its fluvial-lacustrine stratigraphy.
Detailed palynological studies of outcrop samples and well cuttings indicate an
age range for the bulk of Richmond Basin sediments as early Carnian to late
middle Carnian (early Late Triassic).
Seven new genera
and thirteen new species are described from the deltaic-lacustrine Vinita Beds
of the Richmond Basin, including a new zone fossil for the early-middle
Carnian, Placopollis Koobii n. gen. et sp. Six new genera and eleven new
species of angiosperm-like pollen are recognized: Dicrinopollis operculatus
n. gen. et sp., Monocrinopollis doylei, n. gen. et sp., M.
microreticullatus n. sp., M. mulleri n. sp., M. walkeri n.
sp., Pentecrinopollis gemmatus n. gen. et sp., P. traversei n.
sp., Polycolpopollis magnificus n. gen. et sp., Tricrinopollis
minutus n. gen. et sp., P. olsenii n. sp., and Zonacrinopollis
anasulcatus n. gen. et sp. Most of the angiosperm-like species are placed
in the Crinopolles Group, which is also defined. Steevesipollenites
STOVER is emended to include S. hemiplicatus n. sp., which is
related to the Crinopolles Group as a possible polyplicate precursor. The
stratigraphic record of this group is traced from its first appearance below
the Vinita Beds to the migration of M. microreticulatus n. sp. into younger
Triassic strata of eastern and western North America. The morphological and
evolutionary implications of Triassic angiosperm-like pollen are discussed and
reconciled with recent cladistic analyses of angiosperm - seed plant
relationships.
Key words: Pollen;
angiosperm-like; crinopolles; Late Triassic; rift basin.
Table of Contents
|
Introduction.......................................................................................... Richmond Basin Geology....................................................
...........
... Richmond Basin Palynology..................................................
.
........ Richmond Basin Age...................................................
..
...... Materials and Methods......................................................
..
....... Systematic Palynology....................................................
........
The Crinopolles Group.......................................................
..
....... Pentecrinopollis n.
gen..............................................................
Tricrinopollis n. gen. ..................................................
...............
Monocrinopollis n. gen................................................
...............
Dicrinopollis n. gen....................................................
.
...........
Zonacrinopollis n. gen...............................................................
Polycolpopollis n. gen
.....
...................
Steevesipollenites emend
.
.....................
Placopollis n. gen
........................ Discussion
..
....................
Origin
and Evolution of the Crinopolles Group
......................
Habitat
Evolution within the Crinopolles Group
....................
Morphological
Comparisons and Significance
....................
Phylogenetic Significance
......................... Conclusions
...................... Addendum
........................ Acknowledgements
.................. Literature Cited
.................. Explanation of Plates .. .................... |
38 38 41 42 44 45 46 46 49 54 62 64 65 68 69 70 70 73 74 77 80 81 81 81 84 |
Over the past eighteen years considerable fossil data have been brought to bear
on the problem of angiosperm origin and early evolution. Unlike most
investigations into the early history of angiosperms, this investigation began
with the discovery of angiosperm-like pollen in rocks at least 100 million
years older than previously dated angiosperm pollen (CORNET 1977; 1979). Most
investigators concentrated their search in Lower Cretaceous rocks, where they
hoped to find an answer to the long standing mystery of angiosperm origin. Out
of their search came new evidence for a major mid-Cretaceous radiation of
primitive dicots (DOYLE & HICKEY 1978), but as hard as they looked, they
could not find any clues to angiosperm ancestry. Instead, their investigations
showed that angiosperm pollen precedes the record of angiosperm leaves in the
Lower Cretaceous, and that angiosperm fossils become fewer and harder to find
with age, until they seem to disappear below the Barremian or upper Hauterivian
(DOYLE et al. 1977; DOYLE 1978a; BRENNER 1984; RETALLACK & DILCHER 1981;
1986; BRENNER 1987, Abs.).
Much of the bias in accepted age for the oldest angiosperms stems from the
failure of paleobotanists to "convincingly" demonstrate
pre-Cretaceous angiosperm megafossils (CORNET 1986, notwithstanding). Yet, the
burden of proof for pre-Cretaceous angiosperms has become increasingly more
difficult as belief in a Cretaceous origin has gained strength due more to
popularity than substance (cf. WALKER & WALKER 1984: p. 516). The
"consistent failure of palynologists to find distinctively angiospermous
pollen types in Triassic, Jurassic, and earliest Cretaceous strata" (DOYLE
1977: p. 501) has been more a problem of acceptance than a dearth of evidence.
Rare accounts of Clavatipollenites hughesii, Liliacidites spp.,
or reticulate-columellate tricolpates in the Jurassic (
SCHULZ 1967; HOROWITZ 1970; LUND 1977; POCOCK 1978; CORNET 1981) have
been dismissed or ignored, but never pursued by others with the intent of
testing interpretations of the Cretaceous angiosperm pollen record. BAKKER
(1978: p. 661) prematurely remarked that Late Triassic Newark "rift
valleys...deep in the interior of the Laurasian supercontinent...seem ideal for
trapping remains of...pre-Cretaceous angiosperms... But no trace of angiosperms
has been found among Newark pollen or leaf floras." The palynological
discoveries reported here may represent the oldest clues to the origin and
early evolution of the angiosperms, and are not inconsistent with
interpretations, based on recent cladistic analyses, that the Gnetales,
Bennettitales, and angiosperms are sister groups, having a possible common
origin in the Triassic (DOYLE & DONOGHUE 1986; 1987; CRANE 1985).
The amount of information about the geology of the Richmond Basin has increased
significantly since drilling began in 1980 for oil and gas in the basin. The
author's work on angiosperm-like pollen from the basin was interrupted by his
active participation in drilling in the basin. Two deep test wells were drilled
in 1981 (Horner No. 1 and Bailey No. 1), proving that the basin was more than
twice as deep as previously indicated in the literature (e.g. SHALER & WOODWORTH
1899). To date, at least 17 shallow wells (under 3,000 ft./915
m.) and three deep wells have been drilled in the basin (Text-fig. 1), with four of them reaching
basement at 1190 feet/363 meters (Chalkley No. 1); 2,770 feet/844 meters
(Adamson No. 1); 4,490 feet/1,369 meters J. R. Hicks No. 1); and 7,140
feet/2,177 meters (Bailey No. 1).
The structural history of the basin, based on the study of seismic and well
data (CORNET & ZIEGLER 1985), is much more complicated than previously
thought. Carnian age sediments lie unconformably upon metamorphic and igneous
basement (lower unconformity, Text-fig. 2).
A syndepositional unconformity (middle unconformity, Text-fig. 2) separates an
underlying sequence consisting of about 3,200 feet/975 meters of folded,
faulted, and rotated deltaic-lacustrine sediments (i.e. Vinita Beds, Productive
Coal Measures, and basal Lower Barren Beds: SHALER & WOODWORTH 1899) from
an overlying sequence consisting of up to 3,700 feet/1128 meters of
deltaic-lacustrine and fluvio-deltaic sediments. The middle unconformity is
best developed in up-dip areas of rotated fault blocks, where it is angular,
but may disappear in synclines where deposition may have been continuous.
Vinita Beds sediments eroded from the tops of anticlines and steeply-dipping
fault blocks were redeposited in synclinal and fault-controlled lows. Deep
erosional canyons are cut into the youngest fluvio-lacustrine sequence, and filled
with the poorly sorted arkosic and conglomeratic Otterdale Sandstone of
possible Jurassic age (upper unconformity, Text-fig. 2).
Two types of depositional cycles are recognizable in the Richmond Basin wells:
1) Very large upwards-coarsening cycles of approximately 2,750 feet (838
meters) in thickness, consisting of swamp and shallow lacustrine facies
dominated by cryptogam spores in the lowest part, mostly lacustrine facies in
the middle, and fluvial-deltaic facies dominated by pollen in the upper part of
the cycle (palynofloral sequences a, b, c and d: Text-fig. 2), and 2)
smaller-scale upwards-coarsening cycles averaging 92 feet (28 meters) in
thickness (EDIGER 1986; Text-fig. 3). Two complete large scale cycles are
preserved, as well as the top and bottom portions of two others; each large
cycle is composed of about 30 of the smaller cycles. If these cycles are
controlled by orbital forcing as OLSEN (1986) has demonstrated for the Newark
Basin of New Jersey and Pennsylvania, the ratio of 30: 1 comes closest to the
40,000-50,000-year cycle of the obliquity of the earth's axis and the
1.6-million-year cycle recognizable in the Lockatong and Passaic formations of
the Newark Basin.
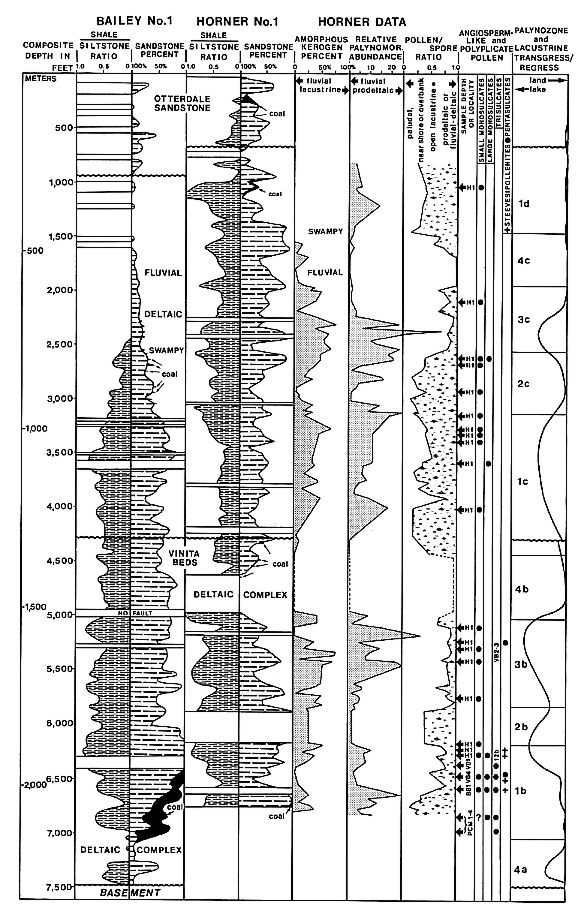
Text-fig.
2. Richmond Basin palynostratigraphy (pollen/spore ratios), kerogen
composition, and paleoenvironmental interpretations in the Horner No. 1 well
for angiosperm-like and polyplicate (Steevesipollenites) pollen occurrences;
lithologic sequences for the Horner No. 1 and Bailey No. 1 wells are correlated
to show dynamic facies changes in basin over a distance of six miles (9.6
kilometers); well lithologies were averaged over 50 ft./15 m. intervals;
lacustrine transgressions and regressions are based on kerogen composition,
fluctuations in palynomorph abundances in sediments, and geophysical log
interpretations and correlations; H1 = Horner well cuttings intervals
containing angiosperm-like pollen; PCMI-4, VB4, VB1, and BB1 = outcrop
localities producing angiosperm-like pollen; three outcrop localities not
containing angiosperm-like pollen (VB2-3, 12b) are also correlated with the
Horner well. This diagram is the original work of the author, and is based on
his own data.
Our understanding of the palynology of the Richmond Basin sequence has greatly
improved over previous knowledge from the study of isolated outcrop localities,
whose stratigraphic relationships could not be tested. The palynofloral history
of the Horner No. 1 reflects the cyclic waxing and waning of large fresh-water
lakes, and the progradation of large deltaic complexes (Text-fig. 2; CORNET
& OLSEN 1985). A graph of the relative percentage of amorphous kerogen
(mostly algal debris) in the Horner samples records major lacustrine cycles as
zones of high amorphous kerogen content (Text-fig. 2: column six). A second graph
(Text-fig. 2: column seven) shows the relative abundance of palynomorphs in the
palynological preparations (i.e. number of palynomorphs per traverse of a
slide). That graph tends to track the curve for amorphous kerogen; the
correlation between the curves reflects the finer grain size of the sediments
yielding the higher concentrations of kerogen and palynomorphs. Higher than
normal percentages of palynomorphs occur in prodeltaic facies underlying
prograding deltaic complexes and in swamp facies that lie on top of deltas. A
third graph (Text-fig. 2: column eight) tracks the relative percentage of
pollen versus spores in the Horner No. 1; intervals dominated by spores reflect
either swampy or lacustrine facies, while intervals dominated by pollen reflect
either prodeltaic or fluvio-deltaic facies. Swampy conditions correlate with
the presence of coals, while strictly fluvial conditions correlate with very
low palynomorph abundances, a strong dominance by pollen, and an abundance of
sandstone.
A fourth graph (Text-fig. 2: column 10) gives a summary of lacustrine
transgressions and regressions in proximity to the Horner No. 1 well, based on
the data in Text-fig. 2. Deltas prograding in one part of the basin may be
replaced by lacustrine or prodeltaic facies in another part; the deltaic
complex present in the Horner No. 1 near the top of the Vinita Beds, for
example, is represented by shallow lacustrine and delta-margin deposits with
high palynomorph abundance in the Bailey No. 1 well.
Along with the transgression/regression curve are ten palynozones based on
palynomorph species content (not given). These zones are numbered according to
palynofloral similarity, with palynozone 1 generally being dominated by
cryptogam spores and monosulcate pollen; palynozone 2 having a high percentage
of articulate spores and saccate gymnosperm pollen; palynozone 3 having a high
diversity and abundance of pollen morphotypes; and palynozone 4 being dominated
by a lower diversity of bisaccate and circumsaccate pollen with increasing
spore content in older zones. These sequential facies-controlled palynozones
repeat themselves through the section: Only the top of sequence "a"
is present at the base of the section (Bailey No. 1); sequences "b"
and "C' are relatively complete, indicating little time lost in the Horner
No. 1 at the projected "unconformity" near their mutual boundary;
while sequence "d" is represented only by the spore-dominated lower
zone.
Aratrisporites scabratus, A. fimbriatus, Calamospora nathorstii, and Laricoidites
spp. make up the dominant cryptogam elements of palynozone Ib,
while Osmundacidites spp., Baculatisporites spp. and Granulatisporites
spp. fluctuate in dominance with articulate spores (e.g. Laricoidites
sp. and Pilasporites sp.) in palynozones Ic and Id (CORNET & OLSEN
1985). However, articulate spores strongly dominate the upper half of zone Id.
The percentage of red beds increases upwards in the section, supporting the
changing cryptogam assemblages from ones of high diversity in older strata to
ones of lower diversity near the top of the section. Angiosperm-like pollen is
present throughout most of the palyniferous sections in both the Horner and
Bailey wells (cf Text-fig. 2), but preferentially occurs in near-shore
lacustrine, swamp, and delta-margin facies - an indication that it probably did
not travel far from its source. Angiosperm-like pollen diversity drops
significantly above the middle unconformity, supporting the overall floral
trend of decreasing diversity upwards, and indicating a general preference of
the plants producing angiosperm-like pollen for wetter tropical conditions.
The palynoflora of the Richmond Basin changes in composition more with changes
in facies and climate than with age. Most of the age-diagnostic species persist
throughout the stratigraphic sequence, with the youngest palynoflorules having
more quantitative than qualitative differences from the oldest palynoflorules
(CORNET & OLSEN 1985). Although some reworking is suspected above an angular
unconformity that developed during basin filling (middle unconformity,
Text-fig. 2), younger palynoflorules in the basin come from sediments that
blanket or overlie buried (positive) structure. Overall, the age of the
Richmond Basin sediments (excluding the Otterdale Sandstone) appears to range
from earliest Carnian to late middle Carnian (early Late Triassic), and does
not represent much more than three million years of deposition (DUNAY &
FISHER 1974; CORNET 1977; CORNET & OLSEN 1985; EDIGER 1986). Such a
duration is in agreement with the 65+ obliquity cycles preserved in the basin,
giving a minimum duration of 2.6 to 3.25 million years, depending on whether
the smaller Richmond Basin cycles are 40,000 or 50,000 years in duration (cf
OLSEN 1986). The oldest coal-bearing strata contain the last abundant
appearance of Aratrisporites spp., which has its last acme zone in the
late Ladinian of Europe (EDIGER 1986), while the youngest Triassic sediments in
the basin lose age diagnostic palynomorphs (e.g. Striatoabieites aytugii)
restricted to middle Carnian or older strata in Europe. The presence of Vallasporites
ignacii, Patinasporites toralis/densus, Camerozonosporites rudis,
and Lagenella martinii in the oldest strata of the Richmond Basin (i.e.
Productive Coal Measures and Lower Barren Beds) indicates an age no older than
Cordevolian, or basal Carnian (BRUGMAN 1983). The uniformity in palynofloral
taxa through the section is paralleled by an unchanging fossil fish fauna,
dominated by Dictyopyge spp. (SCHAEFFER & MCDONALD 1978), a
subholostean fish which is not Present in younger
Newark strata containing phytosaur remains. The oldest phytosaurs have been
dated worldwide as late Carnian (CORNET & OLSEN 1985).
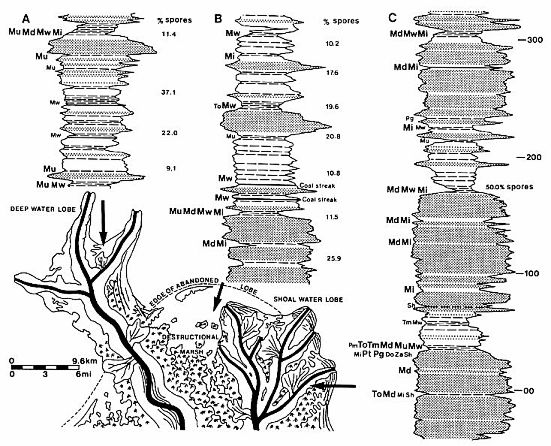
Text-fig.
3. Schematic summary of changing paleoenvironmental distribution of
angiosperm-like and polyplicate pollen taxa in the Richmond Basin, VA. Read
discussion of data quality in Materials and Methods. Some of the repetative
transgressive-regressive lacustrine cycles in the Richmond Basin (gamma ray and
shallow resistivity logs) are used to portray the changing vertical composition
and facies distribution of angiosperm-like pollen through the Vinita Beds.
Representative sequences for three common types of cycles, and their relative
position within a deltaic system; A.
"Shoreface" siltstones and mudstones coarsening upwards into a
bar-finger sandstone (Horner 5,440-5,570'); B. Deltaic plain mudstones, shales, and
thin coal beds with interbedded cravasse subdeltaic sandstones of destructional
marsh sequence on top of an abandoned deltaic lobe (Bailey 4,120-4,350'); C. Three prograding
distributary mouth bar and point bar sandstone cycles within a shoal water lobe
complex (Horner 4,175-4,545'). Sequences B and C correlate with one another as
shown. The distribution of taxa is based on all known occurrences, and does not
represent any particular sequence within a well or outcrop (individual
occurrences are too sporadic or rare). Large TYPE indicates two or more
occurrences in similar parts of analogous depositional cycles, while small type
indicates only one record of an occurrence. Schematic drawing of deltaic system
modified from Galloway (1968). Do = Dicrinopollis operculatus; Md = Monocrinopollis
doylei; Mi = M. microreticulatus; Mu = M. mulleri; Mw = M.
walkeri; Pg = Pentecrinopollis gemmatus; Pm = Polycolpopollis
magnificus; Pt = Pentecrinopollis traversei; Tm = Tricrinopollis
minutus; Po = P. olsenii; Sh = Steevesipollenites hemiplicatus;
Za = Zonacrinopollis anasulcatus. Dot pattern = gray to buff sandstone;
clear pattern = gray to red siltstone and mudstone; dash line pattern = fissile
gray to black shale; horizontal bar = thin coal bed or carbonaceous shale.
Relative percentages of spores are given for these examples of sequences A-C,
with percentages positioned at the top of each 20-30 ft. sample interval.
Vertical scale in 100 ft. intervals.
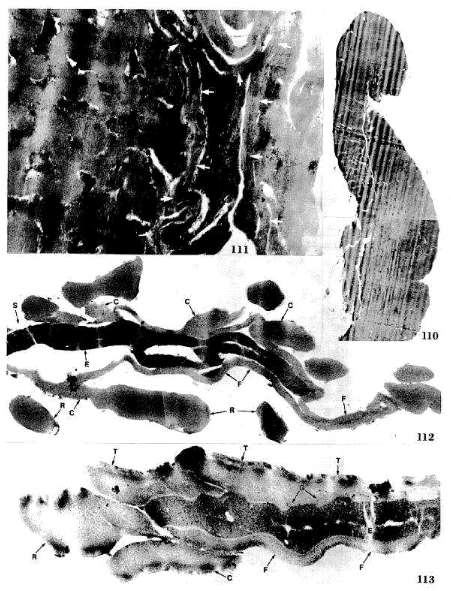
All specimens and holotypes used in the systematic portion of this study come
from outcrop locality VB-4 (Goodwin et al., 1985, Stop 6a) of the Richmond
Basin, Virginia (Text-fig. 1). Strew slides were prepared for outcrop samples
and well cuttings of the basin, and were used for the stratigraphic
distribution and environmental occurrence of described taxa (Text-fig. 2).
Most specimens (including holotypes) used in this study exist as single grain
mounts (279 slides), while the remainder exist as multiple grain mounts (17
slides containing 221 specimens). All single and multiple grain mounts were
picked from strew preparations and photographed using a Zeiss photomicroscope.
Palynological residues were prepared using standard chemical and mechanical
(centrifuge) techniques of rock sample maceration in HF, heavy liquid
separation using zinc bromide, and oxidation with Schultze's solution. The
specimens were stained with Safranin
O.
Lithological, palynological, and kerogen analyses were conducted on cuttings
samples from the Horner No. 1 and Bailey No. 1 wells (Text-figs. 1-2). Electric
log correlations provided the initial means of correlation between the wells,
and along with dipmeter control established the presence of a number of small
faults and at least one large fault crossing each borehole (faults are
represented by horizontal gaps in the lithologic columns: Text-fig. 2);
palynological correlation later substantiated the electric log correlation, and
provided a biostratigraphic basis for interpreting depositional facies.
Sandstone, siltstone, shale, and coal percentages were averaged over 50 foot
cuttings intervals in order to show major facies changes within the wells
(Text-fig. 2). Both palynological and kerogen studies were conducted on the
same cuttings intervals, which ranged from 10 feet to 30 feet.
Palynofacies and environmental interpretations for the sediments producing
angiosperm-like pollen was possible using cuttings because of the separation
and stratigraphic distribution of palyniferous shales and because of the low
percentages of angiosperm-like pollen in any one sample. Upwards-coarsening
depositional cycles, like those reported for the Newark Basin in New Jersey and
Pennsylvania (OLSEN 1986), are the most useful tool for well correlation in the
Richmond Basin. At least 65 such cycles have been identified by the author,
with each containing the darkest organic-rich shales near or at their base
(Text-fig. 3). The cycles range from about 50 to 150 feet (15-45 meters) in
thickness, with organic-rich shale intervals usually separated by more than 30
feet (9 meters) and typically by at least 60 feet (18 meters) of sandstone and
siltstone. Caving during drilling was monitored at lithologic breaks (by CORNET
& WEISBRICH, wellsite geologists), rarely exceeded 10-15% 10 feet (3
meters) below, and rarely persisted in detectable quantities more than 30 feet
(9 meters) below the lithologic break. Caving from higher in the well was
usually less than 5% when it did occur. Since the volume of shale cuttings for
each shale break was usually good, the relative percentage of shale
contamination from higher units was typically diluted to under 5%.
Identifying the shale interval from which angiosperm-like pollen probably came
was relatively simple because the lagged sample interval usually was less than
the spacing of shale beds. Since angiosperm-like pollen abundance was typically
less than 1% and frequently less than 0.5 % of any given sample (1 count in
150-200 palynomorphs), the relative percentage of caved angiosperm-like pollen
would be less than 0.1% (1 count in 1000), making it highly unlikely that caved
specimens were a significant problem in evaluating the stratigraphic
occurrences of angiosperm-like pollen in the wells studied. The environmental
distribution of described taxa was based on all known occurrences (See
Text-fig. 3), because the distribution of individual taxa was sporadic and
rare, and because the repetitive nature of the sedimentary cycles allowed the
identification of the same or analogous environments in each cycle. Text-fig. 3
is an attempt to show 1) the cyclical nature of the fluvial-deltaic facies
within the Richmond Basin, 2) the more frequent occurrence of angiosperm-like
pollen within organic-rich quiet-water shales overlying abandoned delta lobes,
and 3) the changing vertical composition and environmental distribution of
angiosperm-like taxa within the Vinita Beds. Because depositional environments
are repetitive, the most likely explanation for such change is probably biotic
rather than sedimentary.
Systematic Palynology
All palynomorph specimens illustrated herein or used in the systematic part of
this study are deposited in the palynological collections of the U. S.
Geological Survey, National Center, Reston, VA, U. S.
A. The slides containing the holotypes have been appropriately labeled and
designated in the text and plates.
The following list contains all the spore and pollen taxa identified in
palynoflorule VB-4. A comprehensive systematic treatment of the entire
palynoflora has not been undertaken, as most of the recorded taxa (DUNAY &
FISHER 1974; CORNET 1977; CORNET & OLSEN 1985) have been adequately
described and systematically treated elsewhere (SCHULTZ & HOPE 1973; DUNAY
& FISHER 1979; FISHER & DUNAY 1984; EDIGER 1986). Those taxa which are
given detailed systematic treatment are marked with an asterisk.
Alisporites
aequalis
MΔDLER 1964
Alisporites opii DAUGHERTY 1941
Alisporites
ovatus
(BALME & HENNELLY) JANSONIUS 1962
Alisporites
parvus DE
JERSEY 1962
Alisporites
toralis
(LESCHIK) CLARKE 1965
Aratrisporites
saturnii
(THIERGART) MΔDLER 1964
Calamospora
nathorstii
(HALLE) KLAUS 1960
Callialasporites sp. -(cf.
SCHULTZ & HOPE 1973)
Camerosporites
pseudoverrucatus SCHEURING 1970
Camerosporites
secatus
LESCHIK 1955
Colpectopollis cf. C. ellipsoideus
VISSCHER 1966
Converrucosisporites
cameronii
(DE JERSEY) PLAYFORD & DETTMANN 1965
Convolutispora
affulens
(BOLCHOVITINA) SCHULTZ & HOPE 1973
Cornetipollis cf. C. reticulata
POCOCK & VASANTHY 1988
Cycadopites spp. (six unidentified
species)
Deltoidospora
toralis
LESCHIK 1955
Dicrinopollis operculatus
n. gen. et sp.*
Dictyophyllidites
harrisii
COUPER 1958
Duplicisporites
granulatus
LESCHIK 1955
Echinitosporites sp. (new species)
Enzonalasporites
vigens
LESCHIK 1955 emend. SCHEURING 1970
Equisetosporites spp. (Three new species)
Granulatisporites
infirmus
(BALME) CORNET & TRAVERSE 1975
Guthoerlisporites
cancellosus
PLAYFORD & DETTMANN 1965
Laevigatosporites sp.
Lagenella
martinii
(LESCHIK) KLAUS 1960
Laricoidites
intragranulosus BHARADWAJ & SINGH 1963
Lycospora
imperialis JANSONIUS
1962
Microcachryidites
doubingeri
KLAUS 1964
Monocrinopollis
doylei n. gen. et sp.*
Monocrinopollis microreticulatus n. gen. et sp.*
Monocrinopollis mulleri n. gen.
et sp.*
Monocrinopollis walkeri n. gen.
et sp.*
Osmundacidites
senectus
BALME 1963
Ovalipollis
ovalis
KRUTZCH 1955
Paracirculina
scurrilis
SCHEURING 1970
Parillinites
pauper
SCHEURING 1970
Patinasporites
densus
LESCHIK 1955
Pentecrinopollis traversei n.
gen. et sp.*
Pentecrinopollis gemmatus n.
gen. et sp.*
Perotriletes sp.
Pityosporites
devolvens
LESCHIK 1955
Pityosporites
inclusus
LESCHIK 1955
Pityosporites
neomundanus
LESCHIK 1955
Pityosporites
scaurus
(NILSSON) SCHULZ 1967
Placopollis
koobii n. gen. et sp.*
Platysaccus
triassicus
(MALJAVKINA) DUNAY & FISHER 1979
Plicatisaccus
badius
PAUTSCH 1971
Polycolpopollis magnificus
n. gen. et sp.*
Protohaploxypinus sp.
Protohaploxypinus
arizonicus
FISHER & DUNAY 1984
Pseudoenzonalaporites
summus
SCHEURING 1970
Pyramidisporites
traversei
DUNAY & FISHER 1979
Raistrickia
grovensis
SCHOPF 1944
Rugubivesiculites
proavitus
FISHER & DUNAY 1984
Steevesipollenites hemiplicatus sp. nov.*
Striatoabieites
aytugii
VISSCHER 1966
Sulcatisporites
Kraeuselii
MΔDLER 1964
Tetrad type 39
(CORNET 1977: PI. 18, Figs. 5-6)
Triadispora
verrucata
(SCHULZ) SCHEURING 1970
Tricrinopollis olsenii n. gen. et sp.*
Tricrinopollis minutus n. gen. et sp.*
Umbrosaccus
keuperianus
MΔDLER 1964
Vallasporites
ignacii
LESCHIK 1955
Vitreisporites
pallidus
(REISSINGER) NILSSON 1958
Zonacrinopollis anasulcatus
n. gen. et sp.*
At least seventy three palynomorph taxa are present in palynoflorule VB-4 of
the Richmond Basin, VA (Text-fig. 1). Based on a count of 390 palynomorphs,
80.5% are pollen and 19.5% are spores. The assemblage was recorded according to
morphotype (or presumed taxonomic grouping in the case of articulate spores,
lycopod spores, and angiosperm-like pollen):
Striate bisaccates
.................................................................
.....
0.3%
Large bisaccates
(>40 microns) ...........................................
... 40.0%
Small bisaccates
(<40 microns) ...........................................
.... 5.1%
Circumsaccates
(e.g. Patinasporites) ......................................
.. 14.9%
Dispersed tetrads
(e.g. Placopollis)
......................................
. 2.6%
Monosulcates (excl.
angiosperm-like types) ..........................
. 12.0%
Angiosperm-like morphotypes
......
..........................................
. 2.0%
Circumpolles (e.g. Camerosporites)
...........................................
. 3.6%
Articulate spores
(e.g. Laricoidites)
...........................................
.. 0.8%
Lycopod spores
(e.g. Aratrisporites ..........................................
0.5%
Psilate spores
(e.g. Dictyophyllidites) .........................................
.
1.8%
All other
sculptured spores ............................................
....16.4%
Total: 100.0%
The Crinopolles Group
Definition: A group of pollen morphotypes united by their typically (but not
exclusively) reticulate-columellate exine structure, the presence of two or
more sulci (or furrows) which are restricted to the distal and equatorial sides
of a grain, the absence of apertures on the proximal side, and the presence in
most taxa of a dimorphic sculptural pattern which resembles that found in
monocots, especially the Liliaceae and the fossil formgenus, Liliacidites
(DOYLE 1973; WALKER & WALKER 1984). This resemblance does not necessarily
indicate that crinopolles pollen was produced by monocots, or for that matter by
angiosperms. The strong resemblance to monocot pollen, however, probably should
not be ignored or minimized, and is therefore reflected here in a name that
suggests morphological similarity but not affinity.
Etymology:
The Crinopolles Group is designated for fossil pollen grains whose generic
names contain the ending, -crinopollis, which means lily-like pollen.
This name and ending in no way imply affinity, just as crinoids are not
monocots.
Pentecrinopollis n. gen.
Type species: Pentecrinopollis traversei CORNET, n. sp.
Diagnosis: Pollen grains pentasulcate or pentaplicate with usually five sulci
or aperture-like furrows (range 4-6 sulci or furrows) positioned on distal and
equatorial sides of grain, and a non-apertural proximal zone or patch; corpus
oblong or elliptical, rarely round; sulci usually separate, not joined; exine
composed of a nexine bearing large (sexinal) gemmae or large prominent clavae
whose heads are joined either by a reticulum or imperforate tectum; clavae on
ridges separating furrows or sulci arranged in rows; clavae on proximal patch
randomly spaced.
Etymology: Pente - Greek, meaning five; crino - derived from
crinum, Greek, meaning lily, lily-like; pollis - Latin, meaning pollen.
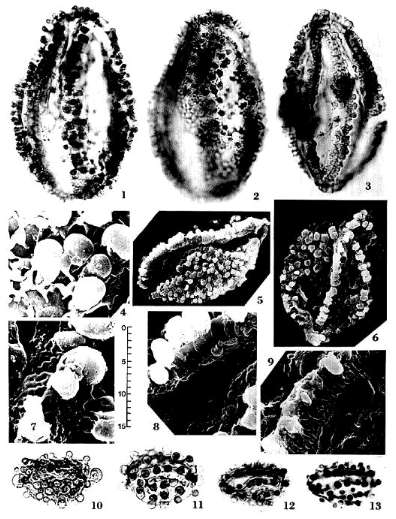
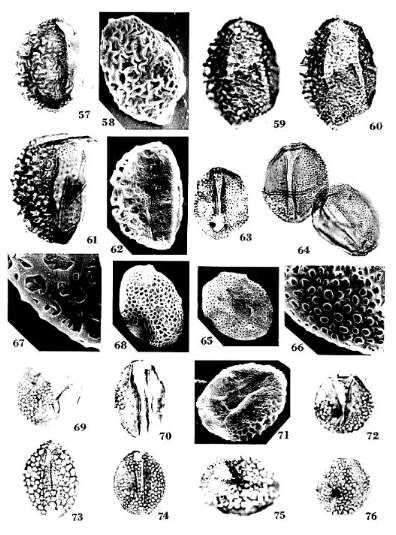
Pentecrinopollis traversei n. sp.
Holotype: SGM-P1, Locality VB4, Richmond Basin, VA,
U.S.A.: pi. 1, Figs. 1-2; dimensions overall 81 X 55 microns.
Diagnosis: Pollen grains pentasulcate with five sulci positioned on distal and
equatorial sides of grain, and a non-apertural proximal zone or patch (Pl. 1,
Figs. 5-6); corpus elliptical to oblong; sulci usually separate, not joined,
each centrally positioned within a broad tapering furrow; ridges separating
furrows about 5-8 microns wide. Exine composed of a 1.0-1.5 micron thick nexine
bearing large prominent clavae whose heads are joined by a delicate reticulum
that abruptly becomes an imperforate tectum along the sides of the furrows containing
the sulci (Pl. 1, Figs. 4; 7-9). Clavae arranged in rows on ridges separating
sulci; clavae crowded and randomly spaced on proximal patch; clavae 2.25-4.75
microns tall; heads shorter than wide, usually 2.25-4.75 microns in diameter;
necks 1.25-2.50 microns tall. Reticulum joins clavae at junction of head and
neck. Non-apertural proximal patch sometimes connected apically at both ends to
the distal ridges by a narrow band of clavate sexine. A pair of equatorial
sulci closely approach one another apically, more so than the three distal
sulci, and their furrows usually join to isolate proximal patch at one or both
ends.
Dimensions (8 specimens): 66-(av. 78)-88 microns in length; 42-(av. 48)-55
microns in width; non-apertural proximal patch about 30-40 microns wide: See Text-fig. 4.
Etymology: traversei - named after Dr. ALFRED TRAVERSE, Professor of
palynology at The Pennsylvania State University, University Park, PA, for his
contributions to our understanding of Triassic palynology and for his support,
both professional and through his NSF grant, during the early phases of this
study.
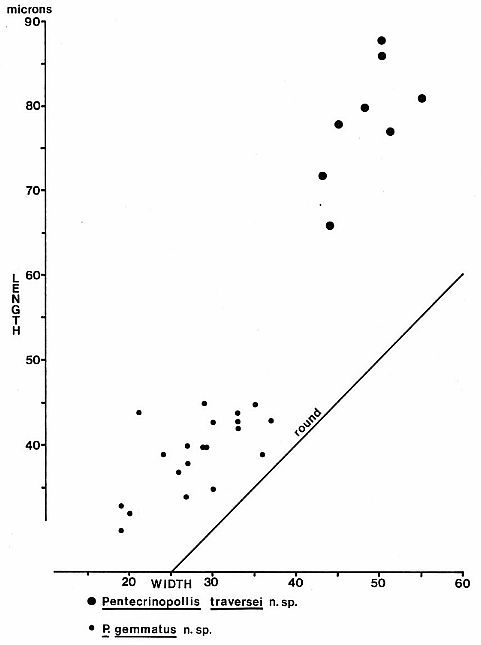
Text-fig.
4. Size distribution in microns for Pentecrinopollis traversei n. sp.
and P. gemmatus n. sp. from palynoflorule VB4.
Remarks: Pentecrinopollis traversei n. sp. possesses all of the
essential characteristics belonging to the Crinopolles Group described in this
paper: multiple sulci restricted to the distal and equatorial sides of the
grain; columellae attached to a footlayer and supporting a reticulate tectum.
The reticulum is attached to the base of the swollen heads of the clavae (Pl.
1, Fig. 4), making the heads supratectal sculptural elements perched on top of
the reticulum. Such a configuration is reminiscent of the crotonoid pattern
found in the monosulcate, Stellatopollis barghoornii DOYLE, from the
Lower Cretaceous (DOYLE et al., 1975). The reticulum of P. traversei n.
sp. is much smaller, however, while the reticulum of S. barghoornii is
organized into large lumina ringed by supratectal elements, which are each
supported by many short columellae rather than one large columella. Just as the
sculptural pattern and large size of Stellatopollis (36-73 microns in
length) is surprising to find in the Lower Cretaceous (DOYLE et al., 1975), the
reticulate-clavate pattern, polysulcate condition, and large size of P.
traversei n. sp. is surprising to find in the Upper Triassic amongst pollen
of the Crinopolles Group whose morphology is more typical of angiosperms.
Age: Late Triassic: Early Carnian.
Occurrence: In the Richmond Basin, P. traversei n. sp. has been recorded
only at outcrop locality VB-4. The relative percentage of P. traversei
n. sp. amongst angiosperm-like pollen in sample VB-4 is 2.4%, or about 0.05%
(5/10,000) of the entire palynoflorule. No specimens were encountered in a
routine slide count of 390 grains, nor in routine counts (usually 100-250
grains) of slides made from cuttings samples in either the Horner No. 1 or
Bailey No. 1 wells (Text-figs. 1-2). One specimen was found, however, in
outcrop locality T20 from near the top of the Falling Creek Member of the
Doswell Formation in the Taylorsville Basin, VA (WEEMS 1980b). The type Falling
Creek Member correlates with the Vinita Beds of the Richmond Basin.
Its pattern of occurrence cannot be determined because of too little data. P.
traversei n. sp. does occur in thin black lacustrine shales overlying
abandoned delta lobes or crevasse splay deposits (See Text-fig. 3 and
discussion in Materials and Methods).
Pentecrinopollis gemmatus n.
sp.
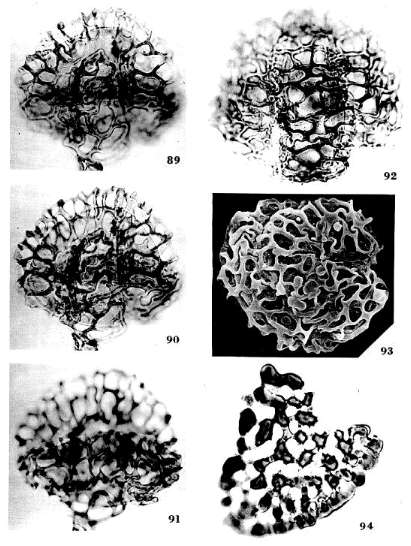
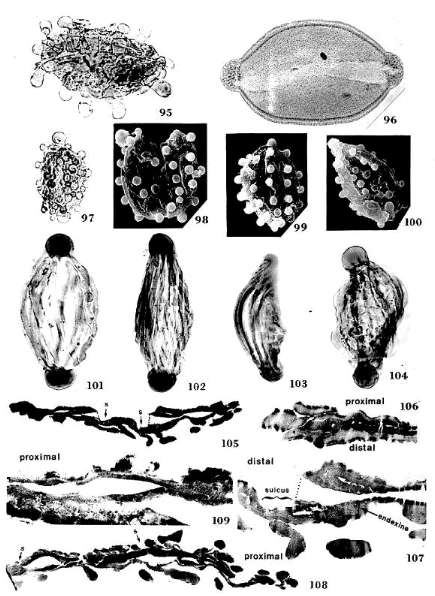
PI. 1, Figs. 10-13; Pl. 8, Figs. 97-100
Holotype: SGM-Q1; Locality VB-4, Richmond Basin, VA, U.S.A.: PI. 1, Figs.
12-13; dimensions overall 32 X 20 microns.
Diagnosis: Pollen grains usually pentaplicate with four to six plications
positioned on distal and equatorial sides of grain, and a non-apertural zone or
patch on proximal side; corpus mostly elliptical, occasionally round. Furrows
shallow, lacking a median rent or sulcus; ridges separating furrows bearing
usually a single row of gemmae; proximal patch protruding slightly (Pl. 1,
Figs. 11; 13) and bearing 7-12 randomly spaced gemmae. Exine about 1.0-1.5
microns thick; tectum either lacking or joined to nexine; surface of exine
between gemmae sparsely scabrate. Gemmae usually 2.25-3.75 microns in diameter,
3.0-4.5 microns tall with constricted bases; an enlarged gemma or auricula,
5.25-9.0 microns in diameter, sometimes present at one or both apical ends of
grain (Pl. 1, Fig. 10; Pl. 8, Figs. 97-98; 100).
Dimensions (20 specimens): 30-(av. 39)-45 microns in length; 19-(av. 29)-41
microns in wide: See Text-fig. 4.
Etymology: gemmatus
- Latin, meaning buds or gems.
Remarks: P. gemmatus n. sp. is included in Pentecrinopollis,
because it possesses the basic construction of P. traversei n. sp., even
though it lacks a reticulum and has furrows instead of sulci. The generic
diagnosis accepts such variation, because 1) P. gemmatus n. sp. is
closer in morphology to P. traversei n. sp. (compare Text-figs. 7A; 7B)
than it is to Steevesipollenites hemiplicatus n. sp., with which it also
compares, and 2) P. gemmatus n. sp. and P. traversei n. sp. are
close enough morphologically to have been produced by related plants. The
occasional presence of auriculae or enlarged gemmae on P. gemmatus n.
sp. provides a link or morphological bridge between P. traversei n. sp.
and S. hemiplicatus n. sp., whose variation includes forms that approach
P. gemmatus n. sp. (e.g. Pl. 8, Fig. 104). The absence of a reticulum
and the presence of polyplicate furrows and occasional auriculae in P.
gemmatus n. sp. are characters interpreted here as less derived than the
presence of a reticulum and sulci, and the absence of auriculae.
Similar morphotypes have been recorded amongst angiosperms: Disulcate
(operculate) clavate pollen resembling P. gemmatus n. sp. was recorded
by G. R. FOURNIER in the Eocene (Pl. 8, Fig. 95); this morphotype probably came
from a monocotyledonous plant (cf. THANIKAIMONI 1970), whose relatives produced
normal reticulate-columellate pollen. Auriculae are present on pollen of some
extant angiosperms (e.g. Bomarea lycina: Amaryllidaceae: P1. 8, Fig.
96), even though other members of the same family produce normal
reticulate-columellate pollen, suggesting that the presence of auriculae is not
a valid reason for excluding P. gemmatus n. sp. from the Crinopolles
Group. In addition, the presence of furrows instead of sulci is a variation in
aperture morphology found in the extant Araceae (e.g. Spathiphyllum
spp.: THANIKAIMONI 1969; TREVISAN 1980).
Age: Late Triassic: Early Carnian.
Occurrence: Pentecrinopollis gemmatus n. sp. has been recorded thus far
only in the Richmond Basin. The relative percentage of P. gemmatus n.
sp. amongst angiosperm-like pollen in sample VB-4 is about 6.1% or about 0.12%
(12/10,000) of the entire palynoflorule. No specimens were encountered in a
routine slide count of 390 grains; specimens were found in cuttings interval
5090-5110 ft./1152/1558 m. (two in 69 count) in the Horner No. 1 well, and in
cuttings interval 5370-5410 ft./1634-1649 m. (one in
209 count) in the Bailey No. 1 well (Text-figs. 1-2).
Its pattern of occurrence suggests that the plant producing P. gemmatus
n. sp. may have preferred fluvial and deltaic sandstone and levee environments,
because it is restricted to thin shales interbedded within deltaic sandstones,
and to black lacustrine shales overlying abandoned delta lobes and crevasse
splay deposits (See Text-fig. 3 and discussion in Materials and Methods).
Tricrinopollis n. gen.
Type species: Tricrinopollis olsenii CORNET n. sp.
Diagnosis: Pollen grains normally with one distal sulcus flanked by a pair of
equatorial or lateral sulci oriented nearly parallel to the distal sulcus;
occasionally one equatorial sulcus missing or an additional sulcus present on
distal side; corpus elliptical to oblong, rarely subrounded; sulci separate,
not joined. Sculpture differentiated, finely reticulate or foveolate distally
and coarsely reticulate proximally; sulci all located within area of finely
reticulate-foveolate distal sculpture; muri of reticulum psilate. Proximal
exine thicker with a footlayer supporting well-developed columellae; columellae
much reduced to granules and short rods on distal side where footlayer absent;
endexine present, continuous, not poly-laminated as in gymnosperms, and
noticeably thicker under sulci or where footlayer absent.
Etymology: Tri - Greek, meaning three, in reference to three sulci; crino
- derived from crinum, Greek, meaning lily, lily-like; pollis - Latin,
meaning pollen.
Remarks: Tricrinopollis n. gen. is very different from Eucommiidites
ERDTMAN 1948, which was once thought to represent pre-Cretaceous angiosperm
pollen, but which is now recognized as gymnospermous (See DOYLE et al., 1975).
The reticulate-columellate exine, lack of obvious endexinal laminations, and
dimorphic sculpture of T. olsenii n. sp. indicate no close relationship
with Eucommiidites, which is absent from most Newark Supergroup
palynofloras. Trisulcate Pretricolpipollenites DANZΙ-CORSIN &
LAVEINE 1963 is present in the Richmond Basin palynoflora, but differs from
Tricrinopollis n. gen. by having an imperforate psilate sculpture and thin
faintly intra-punctate exine with two lateral sulci positioned on the distal
rather than equatorial side (i.e. closer to the median sulcus: BALME 1970). No
intermediate morphotypes were encountered that would suggest a derivation from Pretricolpipollenites,
but intermediates (cf. Text-fig. 7) were found that suggest a derivation from
other morphotypes of the Crinopolles Group. Tricrinopollis n. gen. compares
with trisulcate variants of normally tricolpate Nelumbo pollen (extant
Nymphaeidae: KUPRIANOVA 1979). When Nelumbo pollen is trisulcate, its
tetrad arrangement is tetrahedral, as it is for 7: olsenii n. sp.
Tricrinopollis olsenii n. sp.
Pl. 2, Figs. 14-24; Pl. 8, Figs. 108-109; PI. 9, Fig. 112
Holotype: SGM-I21; Locality VB-4, Richmond Basin, VA, U.S.A.: Pl. 2, Figs.
17-18; dimensions overall 48 X 30 microns.
Diagnosis: Pollen grains normally with one median distal sulcus flanked by a
pair of equatorial sulci oriented nearly parallel to the distal sulcus;
occasionally one equatorial sulcus missing; corpus elliptical to oblong; sulci
separate, not joined. Proximal exine coarsely reticulate-columellate, 1.9-4.0
microns thick; distal exine foveo-reticulate (Pl. 2, Fig. 23), 0.6-1.1 microns
thick; all apertures restricted to area with foveo-reticulate sculpture.
Proximal columellae 2.0-3.0 microns tall, markedly decreasing in height
equatorially; columellae reduced to granules and short rods under area with
finer sculpture (Pl. 8, Fig. 108). Lumina of proximal reticulum variable in
size and heteromorphic, decreasing in size in equatorial transition zone (Pl.
2, Fig. 24); larger lumina 3.0-10.5 microns in maximum dimension; muri of
reticulum psilate. Exine two-layered with a well-developed footlayer
proximally; distal ectexine much thinner than proximal ectexine; footlayer
0.15-0.21 microns thick proximally, disappears equatorially and distally under
finer sculpture; endexine present, continuous, probably not laminated, and
noticeably thicker under sulci and where footlayer absent (Pl. 8, Fig. 109; Pl.
9, Fig. 112).
Dimensions (37 specimens): 42-(av. 46)-53 microns in length; 25-(av. 29)-39
microns in width: See Text-fig. 5.
Etymology: olsenii - named after Dr. PAUL E. OLSEN, Professor of
geology, Lamont-Doherty Earth Observatory of Columbia University, Palisades,
NY, for his extensive contributions to our knowledge of Newark Supergroup
geology and vertebrate paleontology, as well as for his support and aid in
palynological studies of the Newark Supergroup.
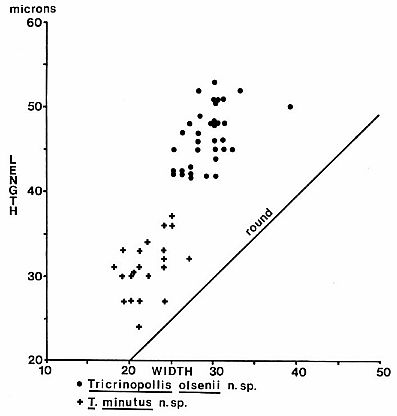
Text-fig.
5. Size distribution inmicrons for Tricrinopollis
olsenii n. sp. and T.
minutus n. sp. from palynoflorule VB-4.
Remarks: The discovery of a tetrad of T. olsenii n. sp. pollen provides
proof that the coarsely reticulate-columellate side is proximal, or facing the
inside of the tetrad, and that the foveo-reticulate side bearing the sulci is
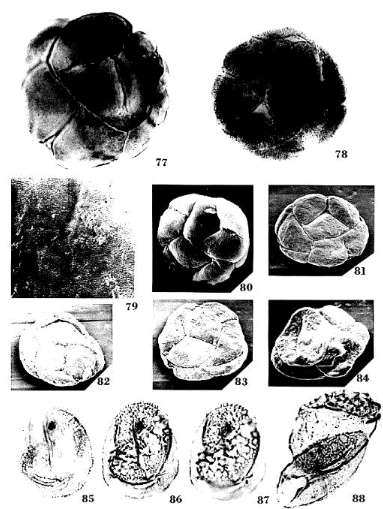
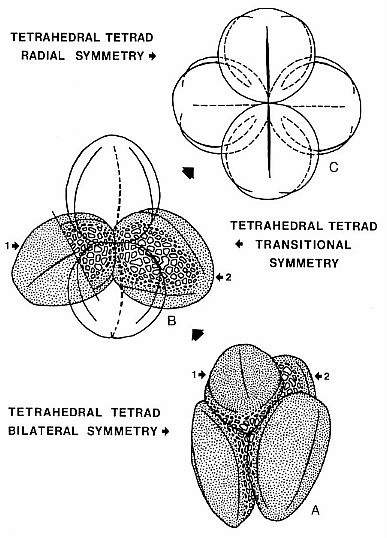
distal (Pl. 2, Figs. 14-16). The tetrad is tetrahedral (Text-fig. 6A), rather
than tetragonal; a tetrahedral arrangement is required for the evolution of
radial symmetry. The polar axis appears to pass through the symmetrical center
of the grain as in monosulcate pollen. The endexine in one TEM cross section
appears to be homogenous (Pl. 9, Fig. 112), while in another (Pl. 8, Fig. 109)
it split in a manner that suggests the presence of a layered structure on the
proximal side of the grain. At best, only two layers can be recognized on the
proximal side, and the outer layer appears to belong to the footlayer (compare
footlayer thickness in Pl. 8, Fig. 109 and Pl. 9, Fig. 112). On the distal side
the endexine is thicker, possibly vacuolated, and clearly not laminated. Since
the endexine of Monocrinopollis doylei n. sp. is similar (possibly vacuolated)
on both proximal and distal sides (Pl. 9, Fig. 13), it may have been undergoing
radical change in the Crinopolles Group.
Age: Late Triassic: Early to middle! Carnian.
Occurrence: Tricrinopollis olsenii n. sp. has been recorded at outcrop
localities BB-1, VB-4, and PCM4 in the Richmond Basin, VA (Text-fig. 1). The
relative percentage of T. olsenii n. sp. amongst angiosperm-like pollen
in sample VB-4 is about 11.3%, or about 0.23% (23/10,000) of the entire
palynoflorule. One specimens was encountered in a routine slide count of 390
grains in palynoflorule VB-4. A specimen was found in cuttings inverval 5800 -
5830 ft./1768 - 1777 m. (one in 112 count) in the
Horner No. 1 well (Text-figs. 1-2). T. olsenii n. sp. also occurs in the
Taylorsville Basin, VA: At outcrop locality T22 (WEEMS 1980a; CORNET 1977:
Doswell) in the Falling Creek Member of the Doswell Formation, and at Locality
T5 (WEEMS 1980b: p. 33, unit 72; CORNET 1977: M'b) located above the type
Falling Creek Member, based on palynological correlation. T. olsenii n.
sp. is found mainly in the Vinita Beds and Productive Coal Measures of the
Richmond Basin, but may range slightly higher into the overlying unit (provided
that it is not reworked).
Its pattern of occurrence suggests that the plant producing T. olsenii
n. sp. may have preferred fluvial and deltaic sandstone and levee environments,
because it is restricted to dark gray to black shales interbedded within thick
fluvial or deltaic sandstones, sometimes containing coal seams, and to black
lacustrine shales overlying abandoned delta lobes and crevasse splay deposits
(See Text-fig. 3 and discussion in Materials and Methods).
Tricrinopollis minutus n. sp.
Holotype: SGM-JI; Locality VB-4, Richmond Basin, VA, U.S.A.: PI. 3, Figs.
36-37; dimensions overall 30 X 20 microns.
Diagnosis: Pollen grains normally with one median distal sulcus flanked by a
pair of equatorial sulci oriented nearly parallel to the distal sulcus;
occasionally equatorial sulci positioned more proximally as in a tricolpate
configuration (Pl. 3, Fig. 41); sometimes an additional sulcus present between
median distal and equatorial sulci (Pl. 3, Figs. 38-39); corpus elliptical to
oblong; sulci separate, not joined. Proximal exine thick, coarsely
reticulate-columellate; distal exine thin with a foveo-reticulate sculpture
(Pl. 3, Fig. 36); all apertures restricted to area with foveo-reticulate
sculpture. Proximal columellae 0.8-1.5 microns tall, markedly decreasing in
height equatorially; columellae much reduced or absent on distal side. Lumina
of proximal reticulum relatively uniform in size except in equatorial
transition zone, where lumina decrease in size; lumina relatively uniform in
shape: pentagonal to rounded, sometimes irregular; larger lumina 1.5-4.5
microns in maximum dimension; muri of reticulum psilate. Exine two-layered with
a well-developed footlayer proximally; endexine probably present but not
observed.
Dimensions (21 specimens): 24-(av. 31)-38 microns in length; 19-(av. 22)-27
microns in width; see Text-fig. 5.
Etymology: minutus- Latin, meaning little, small.
Text-fig.
6. Tetrad symmetry and the possible derivation of tricolpate pollen from
trisulcate pollen; A = Tricrinopollis olsenii n. sp.; B = T. minutus
n. sp.; C = radiosymmetric tricolpate pollen. A trisulcate symmetry similar to
that of T. minutus n. sp. has been noted for Nelumbo pollen
(Kuprianova, 1979); note radial symmetry may be related to pollen shape - the
more round the pollen, the more radial its symmetry and orientation in the
tetrad.
Remarks:
Tricrinopollis minutus
n. sp. forms a population distinct in size from that of T. olsenii n. sp. (Text-fig. 5). This species includes forms that have an
additional anomalous sulcus (i.e. tetrasulcate: Pl. 3,
Figs. 38-39); the anomalous forms provide evidence for patterns of aperture
variation, the origin of multiple apertures, and the direction of aperture
evolution within the Crinopolles Group. If a specimen with five apertures were
found (e.g. Text-fig. 7C), the evolutionary
connection between Pentecrinopollis
and Tricrinopollis
would be easier to envision (Text-fig. 7B-E).
Specimens of T. olsenii
n. sp. have been found in which one of the equatorial sulci is missing (Text-fig. 7H). Another variation in T. minutus n. sp. shows the
equatorial sulci shifting toward the proximal side of the grain (Pl. 3, Fig. 41). Although these forms still possess the
dimorphic sculpture of the genus, the apertures are approaching a tricolpate
configuration (Text-fig. 7F). The orientation of
the grains in the diad also suggests a shift in polarity towards radial
symmetry (Text-fig. 6). These variations
within the genus, Tricrinopollis,
indicate that aperture number and position had not stabilized, and that
variation was occurring is an orderly and systematic fashion. Perhaps that is
why Tricrinopollis
spp. is so short lived. The Richmond Basin appears to have recorded a transient
evolutionary event in which new pollen types, quite unique for the Triassic,
were evolving.
Age:
Late Triassic: Early Carnian.
Occurrence: Tricrinopollis minutus n. sp. has been recorded at outcrop
localities VB-1 and VB-4 in the Richmond Basin, VA (Text-figs.
1-2). The relative percentage of T. minutus n. sp. amongst
angiosperm-like pollen in sample VB-4 is about 6.4 %, or about 0.12%
(12/10,000) of the entire palynoflorule. One specimen was encountered in a
routine slide count of 390 grains in palynoflorule VB-4. No specimens were
encountered in routine counts (usually 100-250 grains) of slides made from
cuttings samples of either the Horner No. 1 or Bailey No. 1 wells (Text-figs. 1-2).
Its pattern of occurrence suggests that the plant producing T. minutus
n. sp. may have preferred fluvial and deltaic sandstone and levee environments,
because it is restricted to thin black shales interbedded within thick fluvial
or deltaic sandstones, and to black lacustrine shales overlying abandoned delta
lobes and crevasse splay deposits (See Text-fig. 3
and discussion in Materials and Methods).
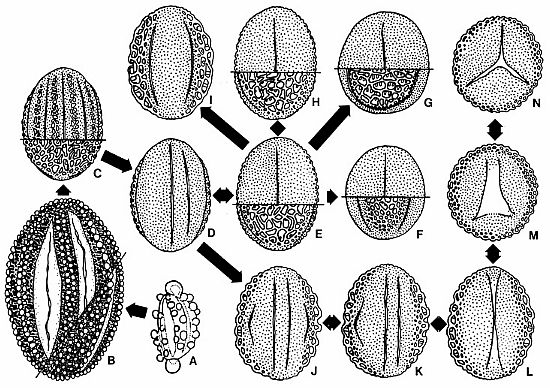
Text-fig.
7. Postulated direction of palynological evolution within the Crinopolles
Group; double-headed arrows indicate variation in same palynomorph species.
This diagram does not represent evolutionary relationships for the parent
plants, which are not known, but does imply polarity (arrows) in aperture and
exine evolution from polyplicate to monosulcate morphology. Also see Text-fig.
12. A = Pentecrinopollis gemmatus n· sp·; B = P. traversei n.
sp.; C = hypothetical intermediate only one step removed from tetrasulcate
variant of Tricrinopollis minutus n. sp. (D); DF -t H = Tricrinopollis
spp.; G = Zonacrinopollis anasulcatus n. sp.; H = T. minutus n.
sp.; I = Dicrinopollis operculatus n. sp.; J-N = Monocrinopollis
spp.; J-K = Monocrinopollis doylei n. sp. and M. mulleri n. sp.
with anomalous apertures; M = Trichotomosulcoid variant and N =
Trichotomosulcate variant of M. doylei n. sp., M. mulleri n. sp.,
and M. walkeri n. sp.
Monocrinopollis
n. gen.
Type species: Monocrinopollis doylei CORNET n. sp.
Diagnosis: Pollen grains usually monosulcate, but with aperture formed by two
closely-spaced sulci separated by a narrow operculum; occasionally an anomalous
sulcus present in equatorial position; corpus oblong to spherical, usually
elliptical; round or spherical specimens usually with a trichotomosulcate
aperture. Proximal exine finely to coarsely reticulate-columellate, distal
exine foveo-reticulate to faintly pitted (almost psilate); all apertures
restricted to area with foveo-reticulate sculpture. Exine two-layered with
well-developed footlayer proximally; ectexine thinner distally with footlayer
discontinuous or missing and columellae reduced to granules and short rods
under area with finer sculpture; endexine, if present, vacuolated,
non-laminated, and thicker under distal aperture.
Etymology: Mono - Greek, meaning one, or a single apertural area; crino
- derived from crinum, Greek, meaning lily, lily-like; pollis Latin,
meaning pollen.
Remarks: The classification of the aperture as monosulcate is based on its
resemblance to the apertures of other monosulcate pollen. If it were not for
the numerous characteristics which link the genus to the Crinopolles Group, the
origin of this type of monosulcus from two closely-spaces sulci probably would
have gone unrecognized (Text-fig. 7J-L).
Whether or not the aperture of other monosulcate grains evolved in a similar
manner is beyond the scope of this paper, but the compound nature of the
aperture is not unique to the Crinopolles Group: The compound distal aperture
is quite common among extant monocots (THANIKAIMON1 1970; CHANDA & GHOSH
1976; CHANDA et al. 1978).
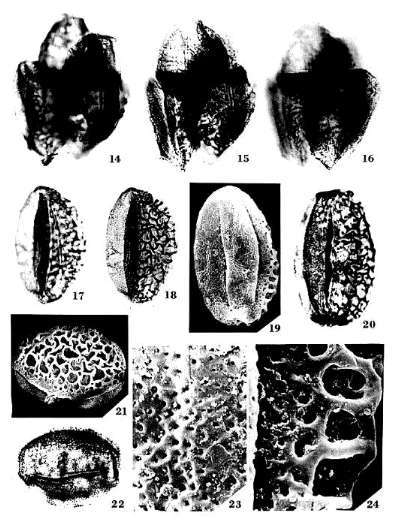
Monocrinopollis doylei n. sp.
PI. 3, Figs. 25-35; Pl. 4, Figs. 53-56; PI. 8, Figs. 105-107; Pl. 9, Fig. 113
Holotype: SGM-A22; Locality VB-4, Richmond Basin, VA, U.S.A.: PI. 4, Fig. 53; dimensions overall 45 X 35 microns.
Diagnosis: Pollen grains usually monosulcate, sometimes trichotomosulcate;
aperture compound, formed by two closely-spaced sulci separated by a narrow
operculum (Pl. 3, Figs. 30-31; Pl.
8, Fig. 105); one or two anomalous sulci occasionally present in equatorial
position (Pl. 3, Figs. 25; 30); corpus oblong to
spherical, usually elliptical; round or spherical specimens usually with a
trichotomosulcate aperture (Pl. 3, Fig. 32). Proximal
exine coarsely reticulate-columellate, 2.0-3.5 microns thick; distal exine
foveo-reticulate, 0.5-0.7 microns thick; all apertures restricted to area with
foveo-reticulate sculpture. Proximal columellae 1.8-3.0 microns tall, markedly
decreasing in height equatorially; columellae reduced to granules and short
rods under area with finer sculpture (Pl. 9, Fig. 113).
Lumina of proximal reticulum irregular in shape, dimorphic with large lumina
dominant; larger lumina 4.5-12.0 microns in maximum dimension, markedly
decreasing in size in equatorial transition zone; smaller lumina 0.8-2.0
microns in diameter; muri of reticulurn psilate. Exine two-layered with
well-developed footlayer proximally; distal ectexine significantly thinner than
proximal ectexine; footlayer 0.15-0.21 microns thick proximally, discontinuous
or missing distally under finer sculpture; endexine continuous, vacuolated,
non-laminated, and thicker under distal aperture (Pl. 8,
Figs. 106-107; Pl. 9, Fig. 113).
Dimensions (74 specimens): 36-(av. 44)-51 microns in length; 29-(av. 34)-45
microns in width; see Text-fig. 8.
Etymology: doylei named after Dr. JAMES A. DOYLE, Professor of botany,
Univ. of California, Davis, CA, for his contributions to our knowledge of Early
Cretaceous angiosperm pollen and leaf floras, and for new interpretations of an
older anthophyte ancestry via his cladistic work with M. T. DONOGHUE.
Remarks: Monocrinopollis doylei n. sp. forms a population similar in
size to that of T. olsenii n. sp., but it is skewed towards more rounded
forms (Text-fig. 10). The shape of the grain determines the form of the
aperture, as in Placopollis koobii n. sp., with the monosulcus
restricted to elliptical or oblong grains and the trichotomosulcus present on
most (67%) but not all (Pl. 3, Fig. 25) round or nearly round grains. Round
grains account for about 12% of the species population. On rare occasions,
round grains possess a circular aperture (Pl. 3, Fig. 27), which resembles the
aperture of Winteraceae pollen with its border of foveolate distal exine
(WALKER et al., 1983). The trichotomosulcus is not always triradiate (Pl. 3,
Fig. 32), and may be triangular in shape with a central operculum (cf. Pl. 4,
Fig. 49). Dimorphic lumina, a sculpture divided into finer and coarser areas,
and a compound distal aperture are characteristics shared with the monocots
(WALKER & WALKER 1984; DOYLE 1973), particularly the Agavaceae (e.g. Hesperocallis
undulata: ALVAREZ & KΦHLER 1987).
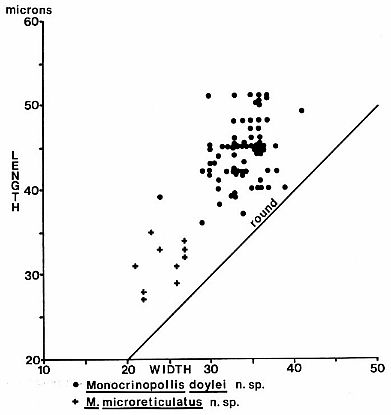
Text-fig.
8. Size distribution in microns for Monocrinopollis doylei n. sp. and M.
microreticulatus n. sp. from palynoflorule VB-4.
Additional
or anomalous sulci are present on about 12 % of the grains; usually only one
reduced or short sulcus is present (67% of the time) in an equatorial position
(Pl. 3, Fig. 30), but occasionally two short sulci are
present (33 % of the time) in equatorial positions (Pl. 3,
Fig. 25). The presence of anomalous sulci indicates that the compound
distal aperture was not derived from equatorial sulci, but probably from a
median distal sulcus and a lateral sulcus, as is occasionally present in T
minutus n. sp. (Pl. 3, Figs. 38-39). In addition,
the compound aperture is sometimes skewed to one side of the distal pole, with
one of its sulci occupying a median distal position (Pl. 3,
Fig. 30; cf. Pl. 4, Fig. 47). These facts and
observations support the theory that aperture variation in the Crinopolles
Group has evolved mainly through the loss of sulci from a prototype or
precursor with at least five distal/ equatorial sulci (like that of Pentecrinopollis
traversei n. sp,: Text-fig. 7). If this theory
is correct, Monocrinopollis n. gen. is not derived from a monosulcate
precursor, but from a polysulcate one. Variation in the form of the compound
distal aperture from monosulcate to trichotomosulcate to round indicates that
aperture morphology and grain shape are closely related. Neither aperture
morphology nor grain shape had stabilized very much in M. doylei n. sp.
over that in Tricrinopollis n. gen., but a trend towards simpler
apertures is already evident. Further explanations for these variations and
their phylogenetic significance are presented in the discussion at the end of
this paper.
Age:
Late Triassic: Early to middle Carnian.
Occurrence:
Monocrinopollis doylei n. sp. has been recorded at outcrop localities
VB-4, BB-1, and PCM-1 (roof shale from coal mine) in the Richmond Basin, VA (Text-figs. 1-2). The relative percentage ofM. doylei n. sp. amongst angiosperm-like pollen in sample
VB-4 is about 22.0%, or about 0.44% (44/10,000) of the entire palynoflorule.
Two specimens were encountered in a routine slide count of 390 grains in
palynoflorule VB-4. In the routine counts of slides made from cuttings samples,
three intervals from the Horner No.1 well yielded specimens (Text-figs. 1-2):
2630-2650
ft./801-808 m. (one in 270 count)
3580-3590 ft./1091-1095 m. (one in 165 count)
5850-5880 ft./1784-1793 m. (one in 116 count)
and three intervals from the Bailey No.1 well yielded
specimens:
4960-4990
ftJ1512-1521 m. (one in 227 count)
5060-5090 ft./1543-1552 m. (one in
230 count)
5470-5500 ft./1668-1677 m. (one in 268 count)
No
specimens have been observed yet in Taylorsville Basin palynoflorules. M.
doylei n. sp. may be an endemic species due to its unusual restriction to
the Richmond Basin, but its distribution may also be due to sampling error.
Its
pattern of occurrence suggests that the plant producing M. doylei n. sp.
may have preferred fluvial and deltaic sandstone and levee environments,
because it is restricted to dark gray to black shales interbedded within thick
fluvial or deltaic sandstones, sometimes containing coal seams, and to black
lacustrine shales overlying abandoned delta lobes and crevasse splay deposits
(See Text-fig. 3 and discussion in Materials and Methods).
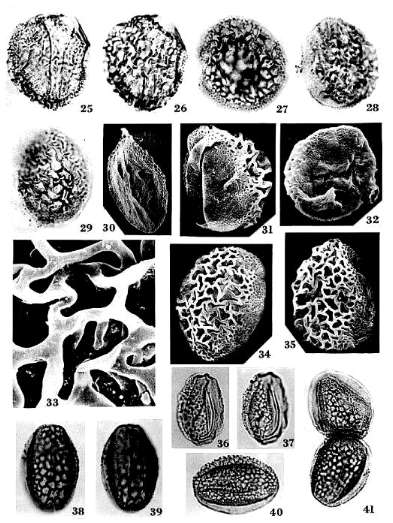
Monocrinopollis mulleri
n. sp.
Holotype:
SGM-C3; Locality VB-4, Richmond Basin, VA, U.S.A.: Pl. 4, Figs. 42-43;
dimensions overall 45X 33 microns.
Diagnosis:
Pollen grains usually monosulcate, sometimes trichotomosulcate; aperture
compound, formed by two closely-spaced sulci separated by a narrow operculum (Pl. 4, Figs. 43-44, 47); one or two anomalous sulci
occasionally present in equatorial position (Pl. 4, Fig. 49);
corpus oblong to spherical, usually elliptical; round or spherical specimens
usually with a trichotomosulcate aperture (Pl. 4, Fig. 49).
Proximal exine coarsely reticulate- columellate, weakly semitectate with some
free-standing columellae under lumina of reticulum; distal exine foveolate; all
apertures restricted to area with foveolate sculpture. Proximal columellae
1.1-1.5 microns tall, markedly decreasing in height equatorially; columellae
reduced or absent under area with finer sculpture. Lumina of proximal reticulum
irregular in shape, ranging from circular to oblong, relatively uniform in
size; larger lumina 1.5-6.0 microns in maximum dimension (most 2.25-5.25
microns wide), markedly decreasing in size in equatorial transition zone; muri
of reticulum psilate. Exine two-layered with well-developed footlayer
proximally; distal ectexine significantly thinner than proximal ectexine;
endexine present, forming a darker inner body in un
oxidized specimens (Pl. 4, Fig. 52).
Dimensions
(39 specimens): 36-(av. 43)-49 ~icrons long; 25-(av. 33)-40 microns wide: See Text-fig. 9.
Etymology:
mulleri - named after the late DR. JAN MULLER for his pioneering and
landmark contributions to our knowledge of the angiosperm pollen record, and
for his candid encouragement of my work on Triassic angiosperm-like pollen and
on Sanmiguelia lewisii (CORNET 1986).
Remarks:
Monocrinopollis mulleri n. sp. forms a population similar in size to
that ofM. doylei n. sp. and 7: olsenii n.
sp., but it contains fewer round or sub-rounded forms than M. doylei n.
sp. (Text-fig. 10). The trichoto- mosulcus is
present on most, but not all, round or nearly round grains. Round grains
account for about 5 % of the species population, which is significantly less
than 12 % for M. doylei n. sp. The trichotomosulcus is not always
triradiate, and may be triangular in shape with a central operculum (Pl. 4, Fig. 49). As for M. doylei n. sp.,
additional or anomalous sulci are present, but on only 5 % of the grains. The
lower percentage of trichotomosulcate forms or forms with anomalous sulci may
indicate more stability in morphology, i. e. a greater degree of
specialization. The relatively smaller and more uniform size of lumina on the
proximal reticulum and shorter columellae compare more with M. walkeri n.
sp. and M. microreticulatus n. sp., which are the smallest and most
widely distributed angiosperm-like species, occurring in the Richmond,
Taylorsville, and Deep River basins. The presence of free- standing columellae
under lumina of the reticulum is a derived character in angiosperms (WALKER
& WALKER 1984). A semitectate exine also occurs in a Norian age species of Liliacidites
from the Newark Basin, PA (CORNET 1977: Locs. M-3, M-4; CORNET & OLSEN
1985: same locality containing Retimonocotpites sp. 173), supporting the
interpretation that M. mulleri n. sp. is more derived than M. doylei n.
sp.
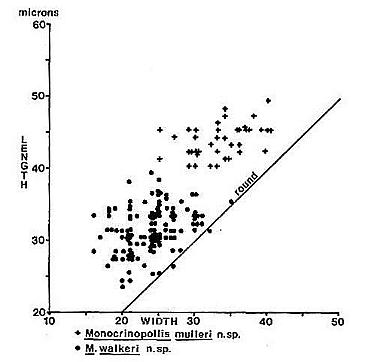
Text-fig.
9. Size distribution in mrcronsfor Monocrinopollis mulleri n. sp. and M.
walkeri n. sp. from palynoflorule VB-4.
Age: Late Triassic: Early to middle Carnian.
Occurrence:
Monocrinopollis mulleri n. sp. has been recorded only at outcrop
locality VB-4 in the Richmond Basin, VA (Text-figs. 1-2).
The relative percentage of M. mulleri n. sp. amongst angiosperm-like
pollen in sample VB-4 is about 12.0%, or about 0.23 % (23/10,000) of the entire
palynoflorule. One specimen was encountered in a routine slide count of 390
grains in palynoflorule VB-4. No specimens were found in routine counts of
slides made from cuttings samples of the Horner No.1 well, but six intervals
from the Bailey No.1 well (Text-figs. 1-2) yielded
specimens:
4230-4260
ftJ1290-1299 m. (one in 77 count)
4300-4330 ft./1311-1320 m.
(one in 78 count)
5410-5440 ft./1649-1658 m. (one in 230 count)
5470-5500 ft./1668-1677 m. (one in 268 count)
5500-5530 ft./1677-1686 m. (one in 198 count)
5560-5590 ft./1695-1704 m. (one in 202 count)
Note
that the top 4100 feet/1250 meters in the Bailey well are mostly barren
sandstones and siltstones. Outcrop locality T20 in the upper Falling Creek
Member and localities T5 and T8 along Stagg Creek in the Taylorsville Basin
have produced specimens of M. mulleri n. sp. (WEEMS 1980b: p. 33-34,
units 72 and 10, respectively). Palynological correlation with the Richmond
Basin section indicates that the section along Stagg Creek does not represent
the basal section in the Taylorsville Basin as indicated by WEEMS (1980b), but
is younger than the type Falling Creek Member in the Taylorsville Basin, which
correlates with the Vinita Beds in the Richmond Basin (Text-fig.
2).
M.
mulleri n. sp. was encountered about as frequently as M. doylei n.
sp. in the Vinita Beds of the Richmond Basin, but it also occurs in the
Taylorsville Basin, whereas M. doylei n. sp. apparently does not. M.
mulleri n. sp. is more common in the southern than northern part of the
Richmond Basin, and may replace M. doylei n. sp. in the Taylorsville
Basin, where it is relatively more common above the type Falling Creek Member.
Its
pattern of occurrence suggests that the plant producing M. mulleri n.
sp. may have preferred delta top and levee environments, because it is most
common in thin dark gray to black shales interbedded within fluvial or deltaic
sandstones, and in black lacustrine shales overlying abandoned delta lobes and
crevasse splay deposits. But unlike Tricrinopollis spp. or M. doylei n.
sp., it also occurs in intradeltaic, clayey siltstone and sandstone
"shoreline" facies (See Text-fig. 3 and discussion in Materials and
Methods).
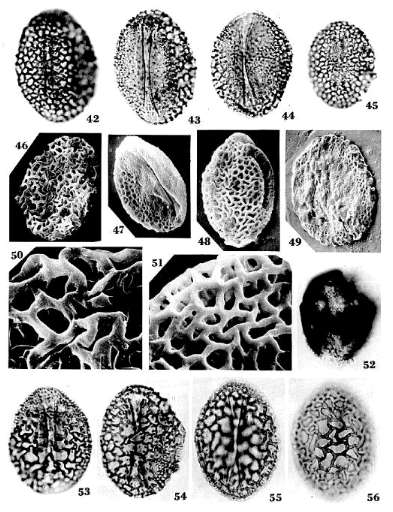
Monocrinopollis walkeri
n. sp.
Pl. 5, Figs. 67-76
Holotype:
SGM-E22; Locality VB-4, Richmond Basin, VA, U.S.A.: Pl. 5,
Fig. 73; dimensions overall 33X24 microns.
Diagnosis:
Pollen grains usually monosulcate, sometimes trichotomosulcoid; aperture
compound, formed by two closely-spaced sulci separated by a narrow operculum (Pl. 5, Fig. 71); anomalous sulci not observed; corpus
oblong to spherical, usually elliptical; round or spherical specimens usually
with a triangular aperture (Pl. 5, Fig. 72). Proximal
exine relatively coarsely reticulate and columellate; distal exine thin,
foveolate (Pl. 5, Fig. 71), and difficult to observe
through proximal reticulum; compound distal aperture symmetrically positioned.
Proximal columellae 0.6-0.7 microns tall, markedly decreasing in height
equatorially; columellae absent under area with finer sculpture. Lumina of
proximal reticulum usually strongly dimorphic; larger lumina mostly regular in
shape, ranging from polygonal to rectangular, and relatively uniform in size;
smaller lumina subcircular (Pl. 5, Fig. 67); larger
lumina 1.1-2.6 microns in maximum dimension (typically 2.3 microns), markedly
decreasing in size in equatorial transition zone; smaller lumina about 0.4-0.5
microns in diameter; muri of reticulum psilate. Exine two-layered with well-
developed footlayer proximally; distal ectexine much thinner than proximal
ectexine; endexine present, frequently forming a darker inner body that
sometimes separates from ectexine.
Dimensions
(94 specimens): 24-(av. 31)-38 microns in length; 16-(av. 24)-35 microns in
width; see Text- fig. 9.
Etymology:
walkeri - named after Dr. jAMES W. WALKER,
Professor of botany, University of Massachusetts, Amherst, MA, and AUDREY G.
WALKER for their joint work on Early Cretaceous angiosperm pollen with
elucidation of character resemblance to the pollen of living dicots and
monocots.
Remarks:
Monocrinopollis walkeri n. sp. forms a population smaller in size than
that of M. doylei n. sp. or M. mulleri n. sp. (Text-figs. 8-9), but similar to that of M.
microreticulatus n. sp. (Text-fig. 10). A
trichotomosulcus is usually not present on round or nearly round grains;
instead, the aperture is either monosulcate or triangular in shape, i.e.
trichotomosulcoid (Pl. 5, Fig. 72). Round grains account
for about 7% of the species population, compared to 12 % for M. doylei n.
sp. and 5 % for M. mulleri n. sp. Additional or anomalous sulci have not
been observed, perhaps because of small grain size. Round grains, however,
sometimes split open along the transition zone between coarse and fine
sculpture (Pl. 5, Fig. 69), indicating an area of weakness there that may be
due to the occurrence of anomalous sulci. M. walkeri n. sp. possesses
dimorphic lumina similar to that of M. doylei n. sp. Lumina size
for M. walkeri n. sp. ranges from relatively large (Pl.
5, Fig. 73) to small (Pl. 5, Fig. 74), approaching
that of M. microreticulatus n. sp. A distinction between the two latter
species appears to exist in that the lumina of M. walkeri n. sp. tend to
be more polygonal in shape, while those of M. microreticulatus n. sp.
are more circular. Some specimens in palynoflorule VB-4 fall at the arbitrary
boundary between these two morphotypes. Average lumina size in M. walkeri n.
sp. appears to decrease in younger strata, making it more difficult to
distinguish the two species.
Dimorphic
lumina, a sculpture divided into finer and coarser areas, and a compound distal
aperture are characteristics shared with the monocots (WALKER & WALKER
1984; DOYLE 1973). The significance of these characters, i. e. whether or not
they are uniquely derived and indicative of affinity, is a subject that will be
of considerable interest to those studying early angiosperm history - particularly since no known
group of gymnosperms produces pollen even remotely similar to that diagnostic
for the monocots. An important distinction between Monocrinopollis spp.
and monocot pollen, however, is the presence of an endexine. Moreover, any
interpretation of affinity must also taken into account that some primitive
dicot pollen still retains an endexine (WALKER & WALKER 1984), while
slightly younger Triassic pollen (Norian, Newark Basin, PA) referrible to Retimonocolpites
and Liliacidites lacks an endexine (CORNET 1977: Pl. 16, figs. 3-5;
Locs. M-3, 4).
Age:
Late Triassic: Early to middle Carnian.
Occurrence:
Monocrinopollis walkeri n. sp. has been recorded only at outcrop
locality VB-4 in the Richmond Basin, VA (Text-fig. 1).
The relative percentage of M. walkeri n. sp. amongst angiosperm-like
pollen in sample VB-4 is about 31.8%, or about 0.64% (64/10,000) of the entire
palynoflorule. Three specimens were encountered in a routine slide count of 390
grains in palynoflorule VB-4. In routine counts of slides made from cuttings
samples, eleven intervals from the Horner No.1 well yielded specimens (Text-figs. 1-2):
1110-1120
ft./338-341 m. (one in 89 count)
2630-2650 ft./802-808 m. (two in 270 count)
2700-2730 ft./823-832 m. (one in 81 count)
2940-2950 ft./896-899 m. (one in 22 count)
3050-3080 ft./930-939 m. (one in 221 count)
3140-3150 ft./957-960 m. (one in 291 count)
3260-3280 ft./993-1000 m. (two in 209 count)
3310-3330 ft./l009-1015 m. (one in 178 count)
3970-3980 ft./1210-1213 m. (one in 129 count)
4960-4970 ft./1512-1515 m. (one in 26 count)
5150-5180 ft./1570-1579 m. (one in 195 count)
5270-5300 ft./1607-1616 m. (one in 272 count)
5610-5640 ft./1710-1720 m. (one in 87 count)
and seven intervals from the Bailey No.1 well yielded
specimens:
4140-4170
ft./1262-1271 m. (one in 206 count)
5680-5710 ft./1732-1741 m. (two in 144 count)
5710-5740 ft./1741-1750 m. (two in 146 count)
5770-5800 ftJ1759-1768 m. (two in 171 count)
5800-5830 ft./1768-1777 m. (one in 112 count)
5860-5890 ft./1786-1796 m. (one in 63 count)
6200-6230 ft./1890-1899 m. (one in 179 count)
Note
that the top 4100 feet/1250 meters in the Bailey well are mostly barren
sandstones and siltstones. Outcrop locality T22 in the upper Falling Creek
Member and localities T5 and T8 along Stagg Creek in the Taylorsville Basin
have produced specimens of M. walkeri n. sp. (WEEMS 1980b: p. 33-34,
units 72 and 10, respectively). Palynological correlation with the Richmond
Basin section indicates that the section along Stagg Creek does not represent
the basal section in the Taylorsville Basin as indicated by WEEMS (1980b), but
is younger than the type Falling Creek Member in the Taylorsville Basin, which
correlates with the Vinita Beds in the Richmond Basin (Text-fig.
2).
M.
walkeri n. sp. is the most abundant angiosperm-like species in the Richmond
Basin, but is more commonly encountered in strata overlying the Vinita Beds
than within them. It appears to be absent from the coal measures underlying the
Vinita Beds, perhaps due to habitat preference or sampling error. Above the
Vinita Beds it is frequently the only angiosperm-like species, other than an
occasional M. microreticulatus n. sp., encountered in routine slide
counts (Text-fig. 2). Similarly, M. walkeri n.
sp. is more common stratigraphically above the type Falling Creek Member of the
Taylorsville Basin than within it.
Its
pattern of occurrence in the Richmond and Taylorsville basins suggests that the
plant producing M. walkeri n. sp. may have preferred delta-margin and
fern-dominated swamp environments (Text-fig. 2),
because it is most common in dark gray to black shales (sometimes containing
abundant fern spbres) interbedded with thin deltaic sandstones, and in black
lacustrine shales overlying abandoned delta lobes and crevasse splay deposits,
but not in shales interbedded within fluvial sandstones containing thick coal
seams (composed mainly of articulate and lycopod remains); M. walkeri n.
sp. also occurs in shallow water interdeltaic clayey siltstones, but it may
have been transported there by lake currents from adjacent swamps (See Text-fig. 3 and discussion in Materials and Methods).
Monocrinopollis microreticulatus n. sp.
Pl. 5, Figs. 63-66
Holotype: SGM-G2; Locality VB4, Richmond Basin,
VA, U.S.A.: Pl. 5, Fig. 64; dimensions overall 34X27
microns.
Diagnosis:
Pollen grains monosulcate; aperture compound, formed by two closely-spaced
sulci separated by a narrow operculum; anomalous sulci not observed; corpus
oblong to elliptical; Proximal exine finely reticulate and columellate; distal
exine thin, psilate to faintly foveolate (Pl. 5, Fig. 64),
and difficult to observe through proximal reticulum; compound distal aperture
symmetrically positioned. Proximal columellae about 0.4-0.6 microns tall,
disappearing on distal side. Lumina of proximal reticulum mostly regular in
shape, ranging from circular to oval, and relatively uniform in size; lumina
0.4-0.8 microns in diameter, markedly decreasing in size in equatorial
transition zone; muri of reticulum psilate. Exine two-layered with
well-developed footlayer proximally; distal ectexine much thinner than proximal
ectexine; endexine present, frequently forming a darker inner body that
sometimes separates from ectexine.
Dimensions:
(10 specimens): 27-(av. 31)-35 microns in length; 21-(av. 24)-27 microns in
width; see Text- fig. 8.
Etymology:
micro - Greek, meaning small, little; reticulatus - Latin,
meaning net-like, nettled; in reference to the size of the proximal reticulum.
Remarks:
Monocrinopollis microreticulatus n. sp. forms a population similar to
that of M. walkeri n. sp. (Text- fig. 10).
Average lengths and widths for both species are the same. Lumina size for M.
microreticulatus n. sp. is the smallest of the Crinopolles Group. Some
specimens in palynoflorule VB-4 fall at the arbitrary boundary between the two
species; this species usually can be identified by the circular instead of
polygonal shape of its lumina, and by muri that are almost as wide as the
lumina. Columellae are too short to be observed clearly with a light
microscope. SCHULTZ & HOPE (1973: Pl. 20, Fig. 15) illustrate a typical
specimen of M. microreticulatus from the Pekin Formation of the Deep
River Basin, North Carolina, but incorrectly identify it. Their photograph
shows a dark endexinal body, which is common for this species. They compare its
reticulum to an alveolar pattern, probably thinking of a cycadalean affinity.
All the specimens that I have observed from their locality are identical to M.
microreticulatus, and conform to the description given here.
Age:
Late Triassic: Early to late Carnian.
Occurrence:
Monocrinopollis microreticulatus n. sp. has been recorded at outcrop
localities VB-4 and BB-l in the Richmond Basin, VA (Text-fig.
1). The relative percentage of M. microreticulatus n. sp. amongst
angiosperm-like pollen in sample VB-4 is about 3.0%, or about 0.06% (6/10,000)
of the entire palynoflorule. No specimens were encountered in a routine slide
count of 390 grains in palynoflorule VB-4. In routine counts of slides made
from cuttings samples, only one interval from the Homer No.1 well yielded a
specimen: 3970-3980 ft./12l0-1213 m. (one in 129
count). Seven intervals from the Bailey No.1 well yielded specimens (Text-figs. 1-2):
4540-4570
ft./1384-1393 ffi. (one in
222 count)
4630-4660 ft./1412-1421 ffi. (two
in 221 count)
4810-4840 ft./1466-1476 ffi. (three
in 218 count)
4870-4900 ft./1485-1494 ffi. (one
in 270 count)
5060-5090 ft./1543-1552 ffi. (one
in 230 count)
5680-5710 ft./1732-1741 ffi. (one
in 144 count)
6200-6230 ft./1890-1899 ffi. (one
in 179 count)
Note
that the top 4100 feet/1250 meters in the Bailey well are mostly barren
sandstones and siltstones. Outcrop iocality T4 in the lower Newfound Member of
the Doswell Formation, and localities T5 and T6 along Stagg Creek in the
Taylorsville Basin (WEEMS 1980b: p. 33-34, units 72 and 66, respectively) have
produced specimens of M. microreticulatus n. sp. Palynological
correlation with the Richmond Basin section indicates that the section along
Stagg Creek does not represent the basal section in the Taylorsville Basin as
indicated by WEEMS (1980b), but is younger than the type Falling Creek Member
in the Taylorsville Basin, which correlates with the Vinita Beds in the
Richmond Basin (Text-fig. 2). This species is
present at the SCHULTZ & HOPE (1973) outcrop locality in the Pekin
Formation, 1.5 miles (2.5 km) north-west of Gulf, North Carolina, in the Deep
River Basin; the samples come from a lense of dark gray shale and claystone on
the south-east side of the Boren Clay Products Company pit, containing numerous
megafossil compressions of ferns, cycads, and cycadeoids (DELEVORYAS 1970;
DELEVORYAS & HOPE 1973; 1975; 1981). LITWIN (1985: p. 134, Pl. XVII, fig.
36) records this species in the upper Carnian Petrified Forest Member of the
Chinle Formation (Petrified Forest National Park, Arizona), where it occurs in
a palynoflorule containing diversified fern spores, and in strata rich in
megafossil ferns and cycadeoids (DAUGHERTY 1941).
M.
microreticulatus n. sp. first occurs in the lower Vinita Beds of the
Richmond Basin. It occurs infrequently through the Vinita Beds and Falling
Creek Member (Taylorsville Basin), being most common in the southern part of
the Richmond Basin. In the northern part of that basin M. microreticulatus n.
sp. is very rare above the Vinita Beds, but in the Taylorsville Basin it
increases significantly in frequency of occurrence in strata overlying the type
Falling Creek Member (along Stagg Creek). In the Pekin Formation of the Deep
River Basin, which is younger than the Richmond and Taylorsville basins (CORNET
1977; CORNET & OLSEN 1985), M. microreticitlatus n. sp. is the
dominant angiosperm-like pollen grain, replacing M. walkeri n. sp.,
which is either very rare or absent. This species is the only crinopolles
member to range outside the Newark Supergroup, but it is not the only
angiosperm-like grain in the Chinle Formation.
Its pattern of occurrence suggests that the plant producing M.
microreticulatus n. sp. may have preferred fluvial overbank and levee
environments, because it is most common in shales and mottled mudstones
(particularly those with evidence for soil and root development) interbedded
with thick fluvial and deltaic sandstones. M. microreti- culatus n. sp.
also occurs in black lacustrine shales overlying abandoned delta lobes and
crevasse splay deposits (delta top facies), in dark gray to black shales
interbedded with thin deltaic sandstones (delta margin/swamp facies), and in
shallow-water low-energy clayey siltstones (interdeltaic facies), indicating
that this plant was probably an opportunist adapted for more than one habitat
(See Text-fig. 3 and discussion in Materials and Methods). Its survival and
migration into younger basins, while most other crinopolles types disappeared,
may be due to this plant's ability to adapt to changing environmental
conditions as the regional climate became drier (CORNET & OLSEN 1985).
Dicrinopollis n. gen.
Type species: Dicrinopollis operculatus CORNET, n. sp,
Diagnosis: Pollen grains disulcate, with two sulci located near lateral or
equatorial sides of grain, separated by a wide operculum; corpus oblong to
elliptical. Proximal exine coarsely reticulate-columellate, distal exine foveo-
reticulate to faintly pitted (almost psilate); both
apertures restricted to area with foveo-reticulate sculpture. Exine two-layered
with well-developed footlayer proximally; ectexine thinner distally with
footlayer discontinuous or missing and columellae reduced or absent under area
with finer sculpture; endexine present.
Etymology: Di - Latin, meaning two, or a pair of sulci; crino -
derived from crinum, Greek, meaning lily, lily-like; pollis - Latin,
meaning pollen.
Remarks:
The paired sulci of this genus are distinguished from the compound aperture of Monocrinopollis
n. gen. by the greater width of the intervening operculum. Furthermore, the
sulci occupy positions near the transition zone between proximal and distal
sculptures, rather than near the distal pole, suggesting an origin different
from that for Monocrinopollis n. gen. It is suggested that Dicrinopollis
n. gen. arose from a morphotype like Tricrinopollis n. gen. through
the loss of the median distal sulcus (compare Text-figs.
7E and 7I).
Dicrinopollis operculatus n. sp.
PI. 5, Figs. 57-62
Holotype: SGM-L2; Locality VB-4, Richmond Basin, VA, U.S.A.:
PI. 5, Fig. 57; dimensions overall 42X 30 microns.
Diagnosis:
Pollen grains disulcate, with two sulci located near lateral or equatorial sides
of grain, separated by a wide operculum that frequently detaches during
fossilization; sulci sometimes joined at one end of grain to form a V-shaped
aperture (Pl. 5, Fig. 57); corpus oblong to elliptical,
rarely round or spherical. Proximal exine coarsely reticulate-columellate,
distal exine foveo-reticulate while operculum foveolate to faintly pitted
(almost psilate); both apertures restricted to area with foveo-reticulate
sculpture. Columellae on proximal side about 1.4 microns tall; lumina of
reticulum irregular and angular in shape, not dimorphic; lumina 3.0-9.8 microns
in maximum dimension, markedly decreasing in size in equatorial transition
zone; muri of reticulum psilate. Exine two-layered with well-developed footlayer
proximally; ectexine thinner distally with footlayer discontinuous or missing
and columellae reduced or absent under area with finer sculpture; endexine
present.
Dimensions (7 specimens): 38-(av. 43)-46 microns in length; 25-(av. 31)-39
microns in width; see Text-fig. 10, small dots.
Etymology: operculatus - derived from operculum, Latin, meaning lid or
cover; in reference to the large operculum separating the two sulci.
Remarks: Dicrinopollis operculatus n. sp. is similar in size to the two
larger species of Monocrinopollis n. gen. (Text-fig.
10, small dots). It can be distinguished from them by an operculum that
frequently detaches, sulci located in a lateral or equatorial position near the
proximal reticulum, and a large irregular reticulum that appears to lack small
lumina (except in the equatorial transition zone). The lateral position of the
sulci, the occasional apical union of the sulci, and the large detachable operculum
suggest that most of the distal side of the grain functioned as one aperture.
This species was not placed in Monocrinopollis n. gen. for reasons given
under the generic description (see Remarks). Grains with similar large distal
apertures composed of a pair of widely-spaced sulci separated by a distinctive
operculum are found in a number of different extant monocot genera (e.g. Xyris,
Xyridaceae; Polianthes, Agavaceae: THANIKAIMONI 1970; ALVAREZ & KOHLER
1987), but such morphotypes in angiosperms could also be derived from a
monosulcate with a compound distal aperture like that of Monocrinopollis n.
gen.
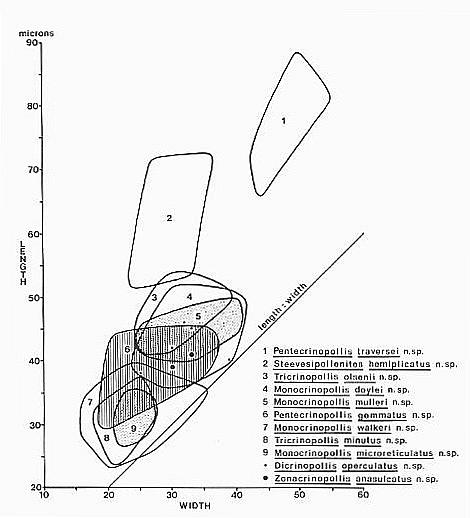
Text-fig.
10. Size-range distribution in microns for Crinopolles Group and Steevesipollenites
hemiplicatus n. sr. from outcrop locality VB4, Richmond Basin, VA. Note
large variation in size between S. hemiplicatus n. sr. and Pentecrinopollis
spp., but the evolution of two distinct size classes for the more derived
members of the Crinopolles Group. Dicrinopollis operculatus n. sr. and Zonacrinopollis
anasulcatus n. sr. are shown as individual specimens because of too little
data for determining population size-range.
Age: Late Triassic: Early Carnian.
Occurrence: Dicrinopollis operculatus n. sp. has been recorded at
outcrop locality VB-4, but may also be present at locality BB-l in the Richmond
Basin, VA (Text-figs. 1-2). The relative percentage
of D. operculatus n. sp. amongst angiosperm-like pollen in sample VB-4
is about 2.0%, or about 0.04% (4/10,000) of the entire palynoflorule. No
specimens were encountered in a routine slide count of 390 grains in
palynoflorule VB-4. No specimens were encountered in routine counts (usually
100-250 grains) of slides made from cuttings samples of either the Horner No.1
or Bailey No.1 wells (Text-figs. 1-2).
Its pattern of occurrence cannot be determined because of too little data. D.
operculatus n. sp. does occur in thin black lacustrine shales overlying
abandoned delta lobes or crevasse splay deposits (See Text-fig.
3 and discussion in Materials and Methods).
Zonacrinopollis n. gen.
Type species: Zonacrinopollis anasulcatus CORNET, n. sp.
Diagnosis:
Pollen grains zonasulculate with an additional sulcus on distal side oriented
parallel to grain's elongate axis; corpus oblong to elliptical. Proximal
reticulum coarsely reticulate and columellate, distal exine foveo- reticulate;
all apertures restricted to area with foveo-reticulate sculpture; zonas ulcus
equatorial (zonizonasulculate), dividing grain into two sub-equal halves. Exine
two-layered with well-developed footlayer proximally; ectexine thinner distally
with footlayer discontinuous or missing and columellae reduced or absent under
area with finer sculpture; endexine probably present, but not observed.
Etymology:
Zona - Greek, meaning belt or girdle; crino - derived from
crinum, Greek, meaning lily, lily-like;pollis -
Latin, meaning pollen.
Remarks:
The zonas ulcus is probably derived from the fusion or union of two equatorial
sulci like those of Tricrinopollis n. gen., because the zonas ulcus is
sometimes incomplete with small strips of exine holding both halves of the
grain together (Pl. 6, Figs. 85-87). The presence of a
median distal sulcus suggests a close relationship with Tricrinopollis n.
gen., but the presence of a ring sulcus indicates a departure from, rather than
an extreme variation of, Tricrinopollis n. gen. Zonasulculate apertures
are also found in primitive dicots (WALKER 1974) and in monocots (THANIKAIMONI
1970). Extant Nelumbo (Nymphaeidae) pollen ranges from tricolpate to
trisulcate with a pair of equatorial sulci positioned as in Tricrinopollis n.
gen. (KUPRIANOVA 1979). The equatorial sulci occasionally join to form a ring
sulcus, as in Zonacrinopollis n. gen.
The
origin of a zonasulcus in angiosperms has usually been attributed to either the
extension or widening of a monos ulcus (DAHLGREN et al., 1985: P. 393;
THANIKAIMONI 1970; WALKER 1974); in some genera aperture variation supports the
circumextension hypothesis, while pollen grains with an equatorial zonasulcus
(perpendicular to the polar axis) support the widening of a monos ulcus to form
an anazonasulcus (WALKER 1974: Fig. 12, 2a-b). The enlargement of an operculum
is thought to be the reason for a ring sulcus shifting to an equatorial
position. But it is the origin and nature of the operculum that is of interest
here, because the half of a zonasulculate grain derived from the operculum
usually has a similar exine structure as the opposite or proximal half. In
other words, it is unlike the aperture membrane of monosulcate gymnosperm
pollen. If the monosulcate aperture in angiosperms had a compound origin as
suggested by the tetrasulcate pollen of Calectasia cyanea (extant
Xanthorrhoeaceae: CHANDA et al., 1978; Calectasiaceae: DAHLGREN et al., 1985),
aperture variation in the Crinopolles Group would have special significance in
the origin of angiosperm pollen.
Zonacrinopollis anasulcatus n. sp.
Pl. 6, Figs. 85-88
Holotype: SGM-Kl; Locality VB-4, Richmond Basin, VA, U.S.A.: PI.
6, Figs. 85-87; dimensions overall 41X33 microns.
Diagnosis:
Pollen grains zonasulculate with an additional sulcus on distal side oriented
parallel to grain's elongate axis; corpus oblong to elliptical. Proximal
reticulum coarsely reticulate and columellate, distal exine foveo- reticulate;
all apertures restricted to area with foveo-reticulate sculpture; zonas ulcus
equatorial (zonizonasulculate), dividing grain into two sub-equal halves;
halves sometimes joined by thin strips of exine that divide zonas ulcus (Pl. 6, Figs. 85-87), otherwise not joined, with exine
tending to split along zonas ulcus (Pl. 6, Fig. 88).
Columellae on proximal side about 2.2 microns tall; lumina of reticulum
irregular and polygonal in shape, not dimorphic; lumina 4.5-7.5 microns in
maximum dimension, markedly decreasing in size in equatorial transition zone;
muri of reticulum psilate. Exine two-layered with well-'developed footlayer
proximally; ectexine thinner distally with footlayer discontinuous or missing
and columellae reduced or absent under area with finer sculpture; endexine
probably present, but not observed.
Dimensions
(2 specimens): 39-41 microns in length; 30-33 microns in width; see Text-fig. 10, large dots.
Etymology:
ana - Greek,
meaning up, back, referring to the distal side of pollen grain; sulcatus -
derived from sulcus, Latin, meaning furrow or groove; in reference to the
median distal sulcus.
Remarks:
See remarks under description of genus. Although only two specimens were found,
this morphotype is distinctive enough to warrant a separate generic and
specific classification. The distal sulcus appears to be twisted or slightly
rotated relative to the longer axis of the pollen grain, suggesting a possible
link with Polycolpopollis n. gen.
Age:
Late Triassic: Early Carnian.
Occurrence:
Zonacrinopollis anasulcatus n. sp. has been recorded only at outcrop
locality VB-4 in the Richmond Basin, VA. The relative percentage of this
species amongst angiosperm-like pollen in sample VB-4 is about 0.6 %, or about
0.01 % (1/10,000) of the entire palynoflorule.
Its
pattern of occurrence cannot be determined because of too little data. Z. anasulcatus
n. sp. does occur in thin black lacustrine shales overlying abandoned delta
lobes or crevasse splay deposits (See Text-fig. 3 and discussion in Materials and
Methods).
Polycolpopollis n. gen.
Type
species: Polycolpopollis magnificus CORNET, n. sp.
Diagnosis:
Pollen grains tricolpate to zonasulculate, usually spiraperturate; corpus
spherical to subspherical; two colpi sometimes joined to form an elliptical
ring sulcus on one side of corpus; colpi usually oriented in different (apolar)
directions on opposite sides of corpus; colpi sometimes parallel in
orientation; ectexine reticulate- columellate with columellae attached to a
well-developed nexine or footlayer.
Etymology:
Polycolpo - Greek, meaning more than two colpi; pollis - Latin,
meaning pollen.
Remarks:
This genus is not included in the Crinopolles Group because it does not exhibit
the dimorphic exine sculpture or structure typical of the group, and because it
may be more closely related to polyplicate pollen of the Equisetosporites-Cornetipollis
type than to polyplicate pollen of the Steevesipollenites type
(POCOCK & V ASANTHY 1988). If it is derived from the Crinopolles Group, it
may represent a modification of the Zonacrinopollis morphotype; Polycoipopollis
n. gen. and Zonacrinopollis n. gen. share the ring sulcus and
rotated anasulcus. The derivation of Polycoipopollis n. gen. from a
crinopolles morphotype would require that the distal exine be modified to match
the proximal exine - an important evolutionary step in bridging the
morphological gap between Lily-like pollen and dicot pollen if tricolpate and
polycolpate angiosperm pollen are related to the Crinopolles Group.
Polycolpopollis magnificus
n. sp.
Pl. 7, Figs. 89-93
Holotype:
SGM-Sl, Locality VB-4, Richmond Basin, VA, U.S.A.: Pl. 7, Fig. 92; dimensions overall 81X74 microns.
Text-fig.
11. Variations in the morphology and position of apertures in Polycolpopollis
magnificus n. sp. from outcrop locality VB4, Richmond Basin, VA; camera
lucida drawings of three specimens (A, C, E) with corresponding outlines of
nexines and aperture configuration to their right (B, D, F); note abrupt
termination of reticulum against nexine along colpus margins, and occasional
fusion of two colpi to form a zonasulcus or ring aperture.
Diagnosis: Pollen grains tricolpate to zonasulculate, usually spiraperturate;
corpus circular to sub circular in equatorial outline; two colpi' usually
oriented subparallel to one another and situated on one side of grain, while
third colpus situated on opposite side of grain and usually oriented at right
angles to the other two (Text-fig. 11 C- F).
The two subparallel colpi sometimes join at both ends to form an elliptical
loop or zonasulcus. Occasionally all three colpi oriented in same direction,
but not equidistantly spaced, with two colpi occupying near-equatorial
positions (Text-fig. 11A-B) as in Tricrinopollis
n. gen. Exine two-layered: ectexine composed of a coarse reticulum
supported by sparse but prominent columellae, which join footlayer or nexine;
columellae typically 4-6 microns tall. Muri of reticulum smooth, 0.8-(av. 2)-3
microns wide; lumina oval, elliptical, or irregular in shape, with maximum
length 3-(av. 9)-11 microns. Nexine about 0.8-1.0 microns thick, apparently
single-layered without an endexine. Colpi form visible grooves in nexinal body
with seXiine thinning at colpus margin as reticulum joins footlayer (Pl. 7, Fig. 93).
Dimensions
(8 specimens): Width 69-90 microns; average diameter 76 microns.
Etymology:
magnificus - Latin, meaning noble or splendid.
Remarks:
The variable orientation ofcolpi and their rotated orientations on opposite
sides of the grain is a characteristic of the Equisetosporites pollen
morphotype (thought by some palynologists to be gnetalean: SCOTT 1960), and
spiraperturate pollen in general. The formation of a ring sulcus is
characteristic of spiraperturate pollen in the Scrophulariaceae, Berberidaceae,
and Acanthaceae (FURNESS (1985). FURNESS (1985: p. 316) states, "One
possible functional interpretation of spiraperturate pollen is that it enables
germination at numerous sites. The majority of taxa with spiraperturate pollen
can be divided into two groups based on habitat; those from hot, dry habitats
and those from hot, wet habitats." "It is therefore suggested that
the spiral aperture is an adaptation to extreme environments which enables
germination at numerous sites and thereby increases the germination rate."
Obviously, such a function is dependent on germination on a stigma rather than
within a micropylar chamber. "The origin of the spiraperture is
undoubtedly polyphyletic and has taken place at various times in the
evolutionary history of diverse plant groups, providing a good example of
convergent evolution." (FURNESS 1985: p. 117). But Furness was referring
mainly to diverse groups within the angiosperms, and P. magnificus n.
sp. also possesses an exine sculpture and structure identical to that of
angiosperms.
Age: Late Triassic: Early Carnian.
Occurrence:
Poiycoipopollis magnificus n. sp. has been recorded thus far only in
palynoflorule VB-4. The relative percentage of P. magnificus n. sp.
amongst angiosperm-like pollen in sample VB-4 is 2.4 %, or about 0.05 %
(5/10,000) of the entire palynoflorule. No grains were encountered in a routine
slide count of 390 grains, nor in routine counts (usually 100-250 grains) of
slides made from cuttings samples in either the Horner No.1 or Bailey No.1
wells (Text-figs. 1-2).
Unidentified fragment
Pl. 7, Fig. 94
Diagnosis:
Pollen grain, possibly polycolpate; corpus probably spherical to subspherical.
Sexine with a massive large reticulum support:ed by
short broad columellae; nexine missing and apparently not attached to
columellae. Muri of reticulum 1-3 microns wide, about 3 microns thick; lumina
elliptical to subround, maximum length 3-(av. 6)-15 microns; columellae oblong,
3-(av. 7)-12 microns X 3-(av. 4.5)-6 microns wide, 3-4 microns tall with
tapered or pointed bases.
Dimensions
(1 specimen): 72X 60 microns, a fragment representing a grain estimated to have
been 95 microns in diameter.
Remarks:
The margins of possibly two colpi are present on the fragment, each represented
by the fusing of adjacent columellae to form a thick ridge, which may have
bordered each colpus. The lack of attachment between sexine and nexine is a
characteristic found in some Equisetosporites pollen (personal
observation) and occasionally in angiosperm pollen (e.g. Retimonocoipites
peroreticulatus (BRENNER) DOYLE: WALKER & WALKER 1984). The apparent
lack of attachment between columellae and nexine excludes this taxon from Polycolpopollis
n. gen.
Age:
Late Triassic: Early Carnian.
Occurrence:
Locality VB-4, Richmond Basin, VA, U.S.A.
Steevesipollenites STOVER
1964, emend.
Type
species: Steevesipollenites multilineatus STOVER 1964.
Emended
generic diagnosis: Acolpate, ellipsoidal to fusiform pollen with as few as 6 or
as many as 40 meridional ridges alternating with narrower furrows. Ridges
usually wider than furrows, usually straight but sometimes undulating in width,
occasionally twisted, and failing to extend across apices or poles. Ridges
distributed more or less uniformly around long axis of grain; occasionally some
ridges very short and crowded between normal ridges, otherwise ridges similar
to one another, except when modified on one side of grain. Modified rj.dges
typically beaded or fused into an area of verrucae and gemmae extending
lengthwise between unmodified ridges. Ridges and furrows end abruptly or
converge before reaching the poles. Poles with gently convex granular caps or
solid, subspherical knobs. Exine two-layered; endexine thin, apparently
continuous and adhering closely to the thicker ridge-forming ectexine, which
thins significantly or is discontinuous in the furrows.
Remarks:
This genus is emended to include S. hemiplicatus n. sp., which agrees in
all aspects but one with the original diagnosis: Its ridges are modified on one
side of the grain. The most distinctive and unifying characteristic of this
polyplicate genus remains its conspicuously modified poles, which are
considered to be more important as a generic characteristic than the
completeness of meridional ridges.
Steevesipollenites hemiplicatus n. sp.
Pl. 8, Figs. 101-104
Holotype:
SGM-Vl; Locality VB-4, Richmond Basin, VA, U.S.A.: Pl. 8,
Fig. 101; dimensions overall 65X 34 microns.
Diagnosis:
Acolpate, ellipsoidal to fusiform pollen with as few as 7 or as many as 19
meridional ridges alternating with narrower furrows. Ridges usually wider than
furrows, usually straight but commonly undulating in width, occasionally
twisted, and failing to extend across apices or poles. Ridges distributed more
or less uniformly around half to most of circumference of grain; occasionally
one or two very short ridges crowded between normal ridges, and on one side of
grain 1-4 ridges (i.e. 9 % to 31 % of all ridges) either strongly modified or
replaced by an elongate patch of verrucae or gemmae, which may occupy up to 42
% of grain (nearly all of one side). Modified ridges strongly undulate,
verrucate, or beaded. Ridges and furrows end abruptly or converge before
reaching the poles. Poles with solid, usually subspherical knobs or auriculae,
10-12 microns in diameter (occasionally convex, e. g. 17 microns tallX 8
microns wide). Exine two-layered; endexine thin, apparently continuous and
adhering closely to the thicker ridge-forming ectexine, which thins
significantly or is discontinuous in the furrows.
Dimensions
(9 specimens): S2-(av. 60)-69 microns in length; 22-(av. 28)-34 microns in
width; see Text-fig. 10 for comparison with Crinopolles Group.
Etymology:
hemi - Greek, meaning half;plicatus - Latin, meaning folded; hemiplicatus
- in reference to only one half of the grain being typically polyplicate,
with the other half modified to a greater or lesser extent.
Remarks:
This species differs from more typical examples of Steevesipollenites spp.
by having one to many of its ridges modified into verrucae or gemmae. The
number of modified and unmodified ridges on a grain varies considerably, and
can be expressed as a relationship: No. of modified ridges/No. of unmodified
ridges. For the nine specimens studied, the following pattern was recorded:
1/11, 2/11, 2/17, 3/19, 4/7, 4/9, patch/9, patch/14, and patch/IS. Either few
ridges are modified, or a verrucate-gemmate patch is present on grains with
many unmodified ridges (11-19); those grains with four modified ridges tend to
have the lowest number of unmodified ridges (7-9). This relationship suggests
that the population of S. hemiplicatus n. sp. is bimodal, and that
morphological stability favored either grains with little ridge modification or
grains with a large verrucate or gemmate patch lacking furrows on one side.
Therefore, S. hemiplicatus n. sp. may represent a species intermediate
between normal Steevesi- pollenites spp. and an auriculate hemiplicate
species like Pentecrinopollis gemmatus n. sp.
Steevesipollenites
spp. has been recorded in the Upper Permian Flowerpot Formation of Oklahoma,
south- central North America (CLAPHAM 1970: Pl. 2, fig. 33), the Middle
Triassic Potrerillos Formation (VOLKHEIMER & ZAVATTIERI 1985) and overlying
Middle Triassic Cacheuta Formation, Cuyo Basin, Argentina, southern South
America (L. E. STOVER, personal communications, 1980; JAIN 1968), the
mid-Cretaceous (Aptian) of the Algerian Sahara, north-west Africa (REVRE 1973:
as Auriculiidites laevigatus), the mid-Cretaceous (middle Albian to
lower Cenomanian) of Brazil in eastern South America (HERNGREEN 1974), and the
mid-Cretaceous (late Albian- Cenomanian/Turonian) of Portuguese Guinea,
Senegal, and Nigeria in West Africa (STOVER 1964; LAWAL & MOULLADE 1987).
S. hemiplicatus n. sp. occurs within the geographic range of the genus,
which extends along a north-south corridor through South America/West Africa
(before continental separation) into south-eastern North America.
Age: Late Triassic: Early Carnian.
Occurrence:
Steevesipollenites hemiplicatus n. sp. has been recorded at outcrop
localities BB-l, VB-4, and l2b in the Richmond Basin, VA (Text-fig. 1). Ediger
(1986: Pl. 26, figs. 2-4) also recorded this species in the basin, but did not
specify the localities. The relative percentage of S. hemiplicatus n.
sp. amongst angiosperm-like pollen in sample VB-4 is about 2.6%, or about 0.05%
(5/10,000) of the entire palynoflorule. No specimens were encountered in a
routine slide count of 390 grains in palynoflorule VB-4. A specimen was found
in cuttings interval 5790-5820 ft./1765-1774 m. (one
in 169 count) in the Horner No.1 well (Text-fig. 2:
+ in column nine). S. hemiplicatus n. sp. was found in the Taylorsville
Basin at the type section of the Falling Creek Member by E. I. ROBBINS (1986,
written communication: Sample 884; ct. WEEMS 1980b), which palynologically
correlates with the lower Vinita Beds of the Richmond Basin.
Its
pattern of occurrence suggests that the plant producing S. hemiplicatus n.
sp. may have preferred fluvial and deltaic sandstone and levee environments,
because it is restricted to thin black shales interbedded within thick fluvial
or deltaic sandstones, and to black lacustrine shales overlying abandoned delta
lobes and crevasse splay deposits (See Text-fig. 3 and discussion in Materials
and Methods).
Placopollis n. gen.
Type species: Placopollis koobii CORNET, n. sp.
Diagnosis: Permanent tetrad; pollen grains monosulcate or typically
trichotomosulcate - variable
within
tetrad; corpus subrounded (if trichotomosulcate) to elliptical (if
monosulcate), with a greatly thickened distal sexine, and a much reduced or
very thin proximal sexine; endexine continuous and laminated; distal sexine
composed of fused granules and rods, sometimes oriented like columellae.
Etymology: Placo - Greek, meaning tablet or plate-like, referring to the
plaque-like portions of the distal exine that are delimited by the apertures
and grain boundaries; pollis - Latin, meaning pollen.
Placopollis koobii
n. sp.
Pl. 6, Figs. 77-84; PI. 9,
Figs. 110-111
Holotype:
MGM-X5, Locality VB-4, Richmond Basin, VA, U.S.A.: PI. 6, Fig. 77; dimensions overall 66 microns in diameter.
Diagnosis:
Permanent tetrad, dispersed tetrad; pollen grains commonly trichotomosulcate
but also monosulcate - variable within tetrad (Pl. 6, Figs.
80-84); corpus subrounded (if trichotomosulcate) to elliptical (if
monosulcate). Distal sexine greatly thickened (about 3-4 microns thick), while
proximal sexine (visible only in cross section) reduced and very thin, except
at proximal pole where grains fused together (about 0..10-0.25
microns thick); endexine laminated (about 0.10-0.13 microns thick) and forming
a continuous inner body (Pl. 9, Fig. 111). Distal
sexine composed of fused granules and rods, which are sometimes oriented
vertically like columellae, other times oriented at random, or fused to form a
massive wall structure; all conditions can exist in the same tetrad (Pl. 9, Fig. 110); distal sexines commonly dominated by one
of these structural types (Pl. 6, Figs. 77-78). Exine
sculpture microscabrate with a thin tectum bearing sparse randomly-spaced small
tectal perforations (Pl. 6, Fig. 79).
Dimensions of tetrad (68 specimens): Diameter 52-93 microns, average 63.4
microns; individual grains range from 36-65 microns in diameter.
Etymology: koobii - named after JOHN D. KOOB, who ineffectively
published this taxon in his 1961 M. S. thesis (Univ. of Massachusetts, p.
35-38) under the name, Placopollis raymondii n. gen. et
sp.
Aperture variation: Tetrads composed of four trichotomosulcate grains are the
most common, while tetrads composed of one or two trichotomosulcate and three
or two monosulcate grains rank second is frequency. Counts of tetrad types were
made from two different localities: VB-4 in the Richmond Basin and T22 in the
adjacent Taylorsville Basin, VA (WEEMS 1980a; CORNET 1977: Doswell); the
percentages of the different tetrad aperture combinations are given below (T =
trichotomosulcate; M = monosulcate):
Locality: VB-4 DOSWELL Combined
|
No. grains: T T T T T T T M T T M M T M M M MMMM |
37 35.2% 5.4% 16.2 % 27.0% 16.2% |
50 40.0 % 14.0 % 22.0% 16.0% 8.0% |
87 37.9% 10.3 % 19.5 % 21.6% 11.4% |
Remarks:
Koob (1961: p. 36-37) did not consider PIacopollis to be a tetrad,
because "the plaque-like units are evidently present only on the outer
surface of the sporomorph". TEM cross sections, however, demonstrate
conclusively that PIacopollis is a tetrahedral tetrad composed of four
proximally fused grains, each with a nexinal body and an unusually thick distal
sexine (Pl. 9, Figs. 110-111). KOOB (1961) gives
dimensions for P. Koobii n. sp. from the Cumnock Formation of the Deep
River Basin, North Carolina, as 50-(60)-70 microns in diameter, which compare
well with measurements from the Richmond Basin. The grains usually appear
psilate or only faintly scabrate under transmitted light as do microscabrate
species of Corollina. EDIGER (1986) considers PIacopollis a
tetrad of "trisaccate" protosaccate grains, with the trichotomosulcus
forming the division between three reduced sacci or
plaque-like units. Such an affinity is possible, since the Richmond Basin
contains two additional and possibly related genera of large dispersed tetrads
whose exines are distinctly alveolar (Tetrad type 39 of CORNET 1977; forms 1,
2, and 4 of EDIGER 1986). KLAUS (1979) illustrates a trichotomosulcate
trisaccate (Dacrycarpites europaeus MΔDLER) from the Middle Triassic,
which may represent the type of pollen grain from which Placopollis evolved.
Age: Late Triassic: Early to early late Carnian.
0ccurrence: P. koobii n. sp. makes up about 2.2 % (22/1000) of
palynoflorule VB-4, and is an important zone fossil, being restricted to the
oldest strata of the Newark Supergroup. P. koobii n. sp. occurs only in
the Richmond, Taylorsville, and Deep River basins (KOOB 1961; CORNET 1977;
CORNET & OLSEN 1985; TRAVERSE 1986). In the Richmond Basin this taxon is
most common in the lacustrine Vinita Beds, decreasing in the underlying coal
measures and in the thick sequence of fluvio-lacustrine strata overlying the
Vinita Beds. Within the Vinita Beds (Text-fig. 2: 4400 ft./1341
m. to 6300 ft./1921 m.) P. koobii ranges in abundance from zero to
16.9%, averaging 3.3% of palynoflorules from the Horner No. 1 and Bailey No. 1
wells (i.e. 95 ten-foot to mostly thirty-foot cuttings samples). Above the
Vinita Beds P. koobii n. sp. has its last occurrence at 2300 ft./701 m. in the Horner No. 1, where its disappearance is
facies controlled. The disappearance coincides with a shift from lacustrine to
fluvial sedimentation near the end of the middle Carnian. P. koobii n. sp.
reappears in the younger Deep River Basin (TRAVERSE 1986), becoming abundant in
the lacustrine Cumnock Formation of early late Carnian age. This taxon
disappears in younger fluvial strata of that basins, apparently becoming
extinct, because it is absent in mid-late Carnian through Norian lacustrine
deposits of the Dan River and Newark basins (ROBBINS 1982; CORNET & OLSEN
1985).
Origin and Evolution of the
Crinopolles Group
The five genera and ten species comprising this morphological group range from
pentasulcates at one extreme to monosulcates at the other. In between these
extremes, aperture variation includes trisulcate, disulcate, and zonasulculate
morphotypes, with the presence of anomalous apertures creating tetrasulcate
variants (Text-fig. 7d; 7J). All of
these morphotypes are united by their similar dimorphic exine structure, with
the apertures being restricted to one side of the grain - typically the side
with a finer foveo-reticulate sculpture and thinner sexine. On the one hand,
the monosulcates are not typically monosulcate, because their apertures are
compound, i.e. composed of a pair of closely-spaced sulci separated by a narrow
operculum; the exine structure of the operculum is similar to that flanking the
compound aperture (e.g. Monocrinopollis doylei n. sp.). The
pentasulcates, on the other hand, are not typically polyplicate (e.g. Pentecrinopollis
gemmatus n. sp.), because one side of the grain lacks furrows, while the
other side shows the development of sulci within the furrows (e.g. P.
traversei n. sp.).
Interpretation of the origin and evolution of the Crinopolles Group is based on
pollen morphology, depositional association, and stratigraphic distribution,
since no megafossil plants have been identified yet as parent plants. In light
of the fact that botanists question the validity of phylogeny based only on
pollen morphology, it needs to be stressed that speculation here about pollen
evolution may be unrelated to any pattern of evolution of the parent plants. Text-figure 7 does not imply that Zonacrinopollis
and Dicrinopollis evolved from Tricrinopollis, or that Pentecrinopollis
traversei n. sp. evolved from P. gemmatus n. sp. Rather, the
morphological relationships of these pollen taxa (i.e. arrows in Text-figure 7) are used to suggest an overall
direction for pollen evolution and a hierarchy of relative morphological
advancement, much in the same manner that DOYLE (1978a) and WALKER (1976)
suggested relative levels of pollen advancement for primitive dicots. This
hierarchy is then tested against stratigraphic distribution to determine if the
"more derived" morphotypes succeed the "less derived" ones.
Such a test, if substantiated, does not prove that the parent plant of Monocrinopollis
walkeri n. sp., for example, is more advanced than that of Tricrinopollis
olsenii n. sp. (consider mosaic evolution), but it would provide a
corollary for consideration.
Aperture evolution (i.e. polarity) within the Crinopolles Group could be in one
of two directions: 1) The monosulcates could be basic,
with the evolution of additional apertures leading to trisulcate and
pentasulcate morphotypes. This interpretation follows conventional thinking
that monosulcate pollen is almost always more primitive than polyaperturate
pollen, particularly if it is present in a phylogenetically related group (e.g.
WALKER 1974; DOYLE 1977; WARD 1986: p. 16; DOYLE & DONOGHUE 1986: p. 421).
If the monosulcates are basic, an evolutionary series could be formed that
starts with Monocrinopollis microreticulatus n. sp., shows a gradual
enlargement of the reticulum and lengthening of columellae through M.
mulleri n. sp. to M. doylei n. sp., adds small equatorial sulci that
elongate to produce the trisulcate forms, and ends with the pentasulcates, the
loss of a reticulate-columellate exine, and the development of polyplicate-like
furrows and apical auriculae.
2) The pentasulcates could be basic, with the trend in aperture evolution from
many to few sulci (Text-fig. 7). This
interpretation regards polyplicate inaperturate (holoaperturate) pollen, such
as Steevesipollenites, as the prototype for or precursor to crinopolles
pollen, and the monosulcate morphotypes as the most derived in the series (Text-fig. 12). Evidence for this
interpretation comes from associated specimens of S. hemiplicatus n. sp.,
which show one side of an auriculate polyplicate grain losing its furrows and
developing gemmae like those on Pentecrinopollis gemmatus n. sp. The
development of sulci within the furrows of P. traversei n. sp. is also
an indication that the pentasulcates are less derived than the monosulcates (Text-fig. 7).
The distribution and number of apertures within the Crinopolles Group, and the
presence of a compound aperture in Monocrinopollis can be best
explained, not by the addition of sulci, but by their loss and modification (Text-fig. 7). If each of the five sulci of Pentecrinopollis
is given a number from one through five (e.g. from left to right), the number
for each sulcus of other crinopolles morphotypes can be determined. This
procedure presumes that sulci did not shift or switch position. In order for
the trisulcates, tetrasulcates, and pentasulcates to evolve from one of the
monosulcates, additional sulci would have to be added. More importantly, there
would be no direct evolutionary route from monosulcate to trisulcate without a
tetrasulcate intermediate (cf. Text-fig. 12):
Sulci numbers one and five would have to evolve de novo, while sulcus
number 2 (or 4) would have to disappear. It seems less likely that new
apertures evolved while others disappeared. A polyplicate origin provides a
template for sulcus evolution and position without the necessity of forcing
crinopolles evolution through a monosulcate prototype.
Additional evidence that the monosulcates are derived comes from the
stratigraphic sequence or distribution of crinopolles pollen in the Richmond
Basin. No angiosperm-like pollen has been found in the oldest strata, i.e. the
Lower Barren Beds (Bailey No. 1 well: 7300 ft., Text-fig.
2), although an abundant diverse palynoflora (63% spores; 37% pollen) was
recovered there. The Richmond Basin contains the oldest near-equatorial
deposits in the Newark Supergroup (CORNET & OLSEN 1985), and introduced a
significant environmental change to this intra-cratonic area with the
development of large lakes and humid peat-forming swamps.
The larger species of each genus are prevalent in the lower Vinita Beds (Text-fig. 2). S. hemiplicatus n. sp. And Pentecrinopollis
spp. are most common in the lower Vinita Beds, decreasing in relative abundance
higher in those beds, and disappearing above them. The pentasulcates are absent
from the underlying Productive Coal Measures, but their absence may be due to
facies control (i.e. different habitat occurrence) or sampling error. Tricrinopollis
spp., Zonacrinopollis sp., and Dicrinopollis sp. are most common
in the lower Vinita Beds, becoming very rare or disappearing stratigraphically
higher. Monocrinopollis doylei n. sp. and M. mulleri n. sp. range higher
than the polyaperturate types, while the smaller species, M. walkeri n.
sp. and M. microreticulatus n. sp., range the highest stratigraphically,
becoming the most common angiosperm-like pollen in the Richmond and
Taylorsville basins. The smaller species are absent below the Vinita Beds
(Text-fig. 2), while M. microreticulatus n. sp. ranges into a younger
Newark Supergroup basin (Deep River Basin: SCHULTZ & HOPE 1973), and ranges
outside the Newark into the late Carnian Chinle Formation of Arizona (LITWIN
1985). Other angiosperm-like species, which closely resemble Monocrinopollis
spp., occur in the Norian (Newark Basin, Pennsylvania: CORNET 1977), indicating
continued survival of this group; these younger taxa can be placed in the
formgenera, Retimonocolpites and Liliacidites (CORNET 1979).
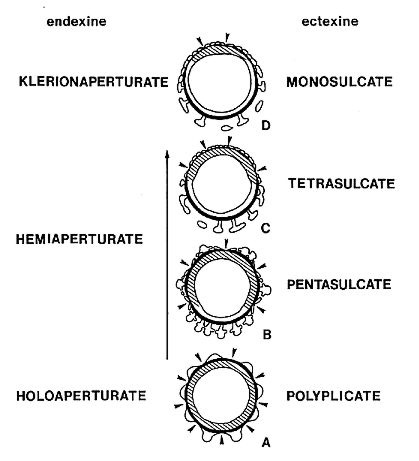
Text-fig.
12. Schematic cross sections of four stages in the hypothetical stepwise
transformation of Steevesipollenites sp. (A) into an angiosperm-like
monosulcate (D) through the loss of furrows and apertural endexine (cross
hatched) on the proximal side, the restriction of apertures (arrows) and loss
of footlayer (black) on the distal side, and the development of a
reticulate-columellate exine tructure; all specimens drawn the same size for
comparison. The distribution of apertural endexine is based on the distribution
of ectexinal apertures, and TEM data for Tricrinopollis olsenii n. sp.
and Monocrinopollis doylei n. sp. Klerion - Greek, meaning partly; hemi
- Greek, meaning half; holo - Greek, meaning entirely. A = Steevesipollenites
hemiplicatus n. sp., B = Pentecrinopollis traversei n. sp., C = Tricrinopollis
minutus n. sp., D = Monocrinopollis doylei n. sp.
The stratigraphic succession of monosulcate, tricolpate, and tricolpate-derived
angiosperm pollen in the Lower Cretaceous is used to support the theory that
radiosymmetric polyaperturate angiosperm pollen is derived from monosulcate
pollen (DOYLE 1977; 1978b). The Early Cretaceous morphotypes are comparable to
forms produced by extant primitive angiosperms (WALKER 1974; WALKER & WALKER
1984), lending credibility to that theory. The monosulcus is, however, largely
a mechanism to prevent desiccation, which is critical for wind pollination
(RETALLACK & DILCHER 1981: P. 46; CRANE 1986: p. 184) but neutral for
biotic pollination. its prevalence among primitive
angiosperms and gymnosperms may be for reasons other than palynological and
genetic conservatism.
The Crinopolles Group is comprised of a close-knit group of morphologically
similar taxa, but appears to be derived from a Middle Triassic polyplicate
(q.v. VOLKHEIMER & ZAVATTIERI 1985): Steevesipollenites extends back to the
Permian, mysteriously disappears in the Jurassic, but reappears again in
tropical or equatorial latitudes during the Aptian-Albian radiation of
Cretaceous anglosperms (CLAPHAM 1970; HERNGREEN 1974). The Crinopolles Group
may have evolved rapidly (punctuated equilibrium?) with the appearance of many
diverse morphotypes closely associated in the same paleoenvironmenta1 facies.
Experimentation in aperture number and position rivals that found in the Early
Cretaceous. Out of this experimentation evolved plants producing small
angiosperm-like monosulcate pollen that adapted and migrated into new areas. A
study of only these small monosulcate morphotypes would yield no clues to their
true origin, if the above thesis on their origin is correct.
Habitat Evolution within the Crinopolles Group
Pentecrinopollis spp., Tricrinopollis spp., Dicrinopollis operculatus
n. sp., Zonacrinopollis anasulcatus n. sp., and Monocrinopollis
doylei n. sp. are all found in either thin dark gray to black shales
interbedded within thick fluvial or deltaic sandstone sequences, or in black
lacustrine shales directly overlying abandoned delta lobes and crevasse splays
(See Text-fig. 3 and discussion in Materials and Methods). The location of the
shales adjacent to active rivers or deltas suggests that the plants producing
these pollen taxa lived mainly in environments adjacent to rivers and
tributaries, but not in basin swamp or lake-shore environments. This particular
group of crinopolles pollen is restricted mostly to the older strata in the
Richmond Basin (Text-fig. 2), and represents the
bulk of experimentation 111 aperture number and position. These taxa occur in
the same or similar strata as Steevesipollenites hemiplicatus n.
sp., which may represent an immigrant from drier upland habitats.
Tricrinopollis olsenii n. sp. and Monocrinopollis doylei n. sp. also occur in
dark gray to black shales associated with thick and extensive coal seams that
are separated by thick fluvial sandstones, suggesting that the plants producing
them ventured more than the others of the above group into coal swamp
environments (cf. FONTAINE 1883; BOCK 1969), possibly following the drainage
systems of the swamp. Monocrinopollis mulleri n. sp., in addition to
occurring in fluvial and deltaic environments, also occurs in silty
"shoreface" environments, particularly above and below bar-finger and
distributary mouth bar sandstones (Text-fig. 3:
sequence A), suggesting that the plant producing this pollen taxon also
inhabited unstable shoreline areas around the large lakes that filled the rift
basin. This additional environment or habitat is supported by the more common
occurrence M. mulleri n. sp. in the southern part of the Richmond Basin
(Bailey No. 1 well), which was always the deeper part of the basin having the
deepest part of any lake. A shift of. M. mulleri n. sp. to include
environments not dominated by fluvial processes is a departure from the norm
for the Crinopolles Group, but so too is its semitectate exine and lower
percentage of morphotypes with anomalous and trichotomosulcate apertures.
Monocrinopollis walkeri n. sp. and M. microreticulatus n. sp. are also found in
depositional sequences different from those of their less-derived
"cousins". M. walkeri n. sp., on the one hand, is found mainly
in delta-margin environments, particularly those dominated by black shales
interbedded with thin (possibly crevasse subdeltaic) sandstones (Text-fig. 3: sequences A; B). The plant producing this
taxon appears to be more common in wet habitats sometimes dominated by ferns
and articulates, suggesting a significant shift in adaptive strategy. M.
microreticulatus n. sp., on the other hand, is found mainly in overbank
shales rich in megafossil plants, including Otozamites spp., Zamites
powellii, Leptocycas gracilis, occasional Macrotaeniopteris
magnifolia, various conifers, and a diversity of ferns (SCHULTZ & HOPE
1973; HOPE & BATTERSON 1969; DELEVORYAS & HOPE 1971; 1975).
The plant producing M. microreticulatus n. sp. seems to have preferred
better-drained soils than the producer of M. walkeri n. sp., since paleosols
and root zones are commonly present where it occurs, and the strata are
typically mottled red and gray with mudstones being more common than fissile
shales. Thus, the smallest monosulcate species of the Crinopolles Group were
produced by plants that became adapted for paleoenvironments represented by the
more common megafossil plant-bearing strata of the Carnian. That shift in
habitat preference may explain why M. walkeri n. sp. and M.
microreticulatus n. sp. are the most common and widespread members of the
Crinopolles Group, but it also explains why M. microreticulatus n. sp.
can be traced into younger Triassic strata dominated by mottled red beds (e.g.
the Painted Desert of Arizona), which contain only occasional thin
fossiliferous gray shales (DAUGHERTY 1941; LITWIN 1985).
How much of this shift in habitat preference is due to evolution, and how much
is due to paleoenvironmental change are questions with no simple answers. The
climate along the East Coast of North America became progressively drier
through the Carnian and Norian (CORNET & OLSEN 1985). The wet coal-forming
environments of the Richmond and Taylorsville basins were replaced by
cyclically drier conditions in younger basins (OLSEN et al., 1978; OLSEN 1986).
Even the coals of the Deep River Basin are not as rich in plant taxa as their
Richmond Basin counterparts (TRAVERSE 1986). The disappearance of the
polysulcate morphotypes may be due to extinction, or the plants producing them
may have simply migrated or survived elsewhere. The survival of a monosulcate
species of the Crinopolles Group into the late Carnian is significant, not so
much because it represents a plant that adapted to a changing climate, but
because its morphology conforms to our concept of primitive angiosperm pollen
(WALKER 1974; DOYLE 1978b; WALKER & WALKER 1984; DOYLE & DONOGHUE 1986:
p. 430).
Morphological Comparisons and
Significance
Pollen of the Crinopolles Group and Tricolpopollis magnificus n. sp.
most closely resemble the pollen of extant angiosperms. No known fossil or
recent gymnospermous pollen possesses a tectate-columellate exine structure in
which the columellae are anchored to an ectexinous footlayer and the endexine
is non-laminated. Columella-like structures are present in some gymnospermous pollen,
such as Mesozoic Corollina pollen (PETTITT & CHALONER 1964; TAYLOR
& ALVIN 1984; CIassopollis is considered a junior synonym: CORNET
& TRAVERSE 1975), and Late Triassic Equisetosporites chinleana (ZAVADA
1984), but in those examples either an ectexinous footlayer is absent or the
columellae are not attached to the nexine. ROWLEY & SRIVASTAVA (1986),
however, describe and illustrate the wall structure of a Corollina torosa
(C. classoides) morphotype from the Oxfordian of Dorset, England, in
which a thin weakly lamellate nexine (i.e. endexinous footlayer or nexine 1 -
not to be confused with the ectexinous footlayer of angiosperm pollen: ZAVADA
1984) is attached to columellae. TAYLOR & ALVIN (1984) present evidence
that the endexine of Corollina is thin and homogenous early in
development, and that lamellae first appear towards the outside of the
endexine, then develop throughout it as the endexine thickens, and finally
become organized into parallel laminations. TEM wall sections of a late Carnian
Equisetosporites sp. (CORNET, personal data, loc. FW6-1: STONE 1978),
like the specimens of C. torosa illustrated by ROWLEY & SRIVASTAVA
(1986), show an intermediate stage in the development of endexinal laminations
with highly contorted and discontinuous lamellae present only in the outer part
of the nexine, a few continuous laminations beneath the lamellae, a thick
homogenous inner nexine, columella-like structures attached to the nexine, and
a thin multilayered footlayer (having a similar grain density as the sexine),
the inner part of which is interbedded with the underlying endexine (POCOCK
& VASANTHY 1988).
These intermediate stages in nexinal development preserved in supposedly mature
dispersed grains of Equisetosporites and Corollina suggest
progenesis, or the early or accelerated maturity of the endexine. Structural
changes possibly caused by early maturation are similar in these unrelated
taxa, and suggest that as laminations are lost columellae are more likely to be
attached to the nexine, an ectexinous footlayer or topographic equivalent
develops (cf. ZAVADA & DILCHER 1988), discontinuous lamellations are
restricted to beneath the footlayer, and the inner endexine remains homogenous
(and possibly porous), as it is during early stages of endexine development.
Furthermore, this modified endexine does not always preserve in the fossil
record (ROWLEY & SRIVASTAVA 1986; ZAVADA 1984). These changes have profound
implications in the interpretation of primitive dicot pollen in which a
multilayered footlayer is present or endexinous lamellations are preserved
beneath the footlayer (e.g. WAHA 1987; WALKER & WALKER 1984; LE THOMAS
& LUGARDON 1975; PRAGLOWSKI 1974). The footlayer of angiosperms may be
derived in part from endexine of gymnosperms - an observation consistent with
lamellations in the footlayer of some angiosperms (TAYLOR et al., 1987: p. 79).
However, not all palynologists consider the endexine of angiosperms to be
homologous with the endexine (footlayer or nexine 1) of gymnosperms, because it
develops after the footlayer (nexine 1) forms in angiosperms (cf. ZAVADA 1984).
DOYLE & DONOGHUE (1986: p. 430) consider the absence of a laminated
endexine together with the presence of columellae (vertical rod-like structures
attached to tectum and footlayer) to be characters derived within the
angiosperms (autapomorphies). But it is also becoming clear that gymnospermous
pollen can approach the angiospermous condition convergently, perhaps for similar
developmental reasons. Since only one group of seed plants is currently known
to produce angiosperm-like pollen (i.e. the Angiospermae), "it would be
premature to rule out the possibility that the parent plants [of Triassic
angiosperm-like pollen] were related to angiosperms but not yet at an
angiospermous level of organization in other organs, or were members of some
unrelated group that evolved angiosperm-like exine structure convergently"
(DOYLE 1978a: p. 367).
TEM sections of crinopolles pollen show no clear evidence of gymnospermous-type
laminations in the endexine. The endexine of T. olsenii n. sp. is
similar to that of M. doylei n. sp., but apertural endexine is thicker
and more extensive, extending under the distal ectexine from one equatorial sulcus
to the other. The endexine of Monocrinopollis doylei n. sp. contains
darkly stained granules that may indicate the presence of small spaces or
canals (Pl. 9, Fig. 113), or the granules may be an indication of
ultra-structural destruction by corrosion (KEDVES 1985). The presence of
laminations in the endexine of PIacopollis koobii n. sp. (Pl. 9, Fig.
111) suggests that palynoflorule VB4 preservation following oxidation is
adequate for endexinal laminations to be preserved, and is good enough that the
sectioning of three crinopolles grains should show laminations in at least one
grain if they existed. In one TEM section of Tricrinopollis olsenii n.
sp. (Pl. 8, Fig. 109) a split nexine creates the illusion of a discontinuous
double-layered endexine on the distal side, and may be either an indication of
weak lamellar development or an artifact of preservation and preparation.
Another specimen of T. olsenii n. sp. (Pl. 9, Fig. 112) preserves an
unbroken exine that does not show any laminations, although the endexine may
have been stained too strongly during preparation to show ultrastructural
detail. The consistent presence of a well-developed ectexinous footlayer,
however, seems to correlate with a non-laminated endexine (see second paragraph
above), and the presence of lamellations in the nexine of some angiosperms and
an internal break parallel to the nexine of T:
olsenii n. sp. only emphasize the parallel unit membrane
construction of the nexine (WAHA 1987).
A problem with preservation was apparent in TEM sections of
tectate-perforate/columellate Magnolia-like pollen from latest Triassic
strata of the Newark Basin, PA (CORNET 1979): One grain had obvious parallel
lamellations preserved in only a portion of the footlayer (although only black
lamellae were present without alternating white lamellae as in the typical
gymnospermous condition), while another grain, presumably of the same species,
showed no lamellations. TAYLOR et al. (1987) found similar variability in Cyclusphaera,
a Barremian-Aptian pollen grain that combines angiosperm and gymnosperm
characters. DOYLE (1984: p. 25), however, prematurely inferred as fact that all
Triassic angiosperm-like pollen had laminated endexines. The majority of all
taxa sectioned show a non-laminated endexine, while some younger Triassic taxa
show no endexine and a thick footlayer as in Retimonocolpites cf. reticulatus
(DOYLE et al. 1975).
In addition to reticulate-columellate exine structure with apparent
non-laminated endexine, there are other characteristics of the Crinopolles
Group which compare with angiosperms, and which may also be synapomorphies
(shared derived characters). The compound distal aperture of Monocrinopollis
spp. is a character shared with the pollen of many extant monocots
(THANIKAIMONI 1970; CHANDA & GHOSH 1976; CHANDA et al. 1978). The
tetrasulcate pollen of extant Calectasia (CHANDA et al. 1978) is
important because it demonstrates that the distal aperture in monocots can and
does sometimes possess two or more distinct sulci separated by operculi with
normal exine structure. By itself, that shared character might be considered an
example of convergence, but crinopolles pollen also possess four additional and
distinctly monocotyledonous characters: 1) The reticulum is differentiated into
finer and coarser areas, with the finer area bordering the distal aperture(s);
2) the coarser reticulum in some species is dimorphic, or differentiated into
small and large lumina; 3) the muri of the reticulum are psilate; and 4) the
non-apertural footlayer is thin (WALKER & WALKER 1984).
The above characters, by themselves, might not carry much taxonomic weight, but
when combined in the same taxa they form a set of synapomorphies shared only
with monocots. The probability that such a distinctive set of characters, i.e.
reticulate-columellate-footlayer exine structure, thin footlayer, psilate muri,
dimorphic sculpture, dimorphic lumina, compound apertures, and
trichotomosulcate apertures could evolve twice in unrelated phylogenies is
unlikely, particularly if such combinations of features have no integrated
function. If they do, then we must look to angiosperms for functional
analogies. The absence of laminations in the endexine is an angiosperm
autapomorphy, but is of little value in monocots, since most extant monocot
pollen seems to lack endexine (WALKER & WALKER 1984; TAYLOR & ALVIN
1984). Exceptions may be noted for Anthurium (Araceae: ROWLEY &
SOUTHWORTH 1967) and Daemonorops (Arecaceae: FREDERIKSEN et al. 1985).
The presence of a thick endexine and the reduction of the distal exine (with
the loss of the footlayer) in the Crinopolles Group indicate that crinopolles
taxa cannot be classified as monocot pollen on palynological criteria, but such
a distinction does not preclude them from having been produced by the direct
ancestors of the monocots or by pre-monocots.
Another comparison between the Crinopolles Group and the monocots comes not
from morphological characteristics, but from paleoenvironmental occurrences.
The plants producing the more primitive pollen of this group may have lived
close to where their pollen is found, i.e. delta top and levee environments or
frequently disturbed habitats. Such habitats are similar to those proposed for
the earliest Cretaceous dicots (HICKEY & DOYLE 1977). The more derived and
persistent pollen of the Crinopolles Group tends to be associated with more
stable swamp and wet floodplain habitats, suggesting a shift in ecology. Such a
shift may have preceded the evolution of the monocots, which today typically
inhabit wetter environments over their dicot relatives.
Population size distribution for the Crinopolles Group suggests that the more
primitive members (i.e. Pentecrinopollis spp.) and Steevesipollenites
hemiplicatus n. sp. were adapted for either an entomophilous or
generalist (ambophilous) pollination syndrome, but not soley an anemophilous
one (Text-fig. 10). The reticulate-clavate exine and large size of P.
traversei n. sp. is particularly indicative of adaptation for insect
transport, while the size range of P.gemmatus n. sp. (30-45 microns) is
within the optimum size range (20-40 microns) for wind pollination (RETALLACK
& DILCHER 1981. p. 45). The gemmate sculpture of P. gemmatus n. sp.,
however, suggests that this taxon was also adapted for entomophily. The ribbed
and otherwise psilate sculpture of S. hemiplicatus n. sp. (52-69
microns) is not necessarily an indication of wind pollination, since
"members of such primitive angiosperm families as the Magnoliaceae,
Degeneriaceae, Eupomatiaceae, and Annonaceae frequently have perfectly psilate
pollen grains and yet are entomophilous" (WALKER & WALKER 1984: p.
516). In addition, the pollen of Ephedra is sticky and transported by
Diptera in search for nectar (BINO et al. 1984).
The more derived members of the Crinopolles Group tend to be clustered in one
of two population sizes: 24 to 40 microns or 36 to 54 microns (Text-fig. 10).
The larger population group includes Tricrinopollis olsenii n. sp., Monocrinopollis
doylei n. sp., and M. mulleri n. sp., all of which have large
reticulate sculptures with tall columellae. This group, because of its size and
sculpture, is better suited for entomophily. The smaller population group
includes T. minutus n. sp., M. walkeri n. sp., and M.
microreticulatus n. sp., all of which have small reticulate sculptures with
short columellae. This group, because of its size, is better suited for
anemophily (cf. CRANE 1986). Reticulate sculpturing is not always an indication
of entomophily in angiosperms (WALKER & WALKER 1984). The
chronostratigraphic persistence and relative abundance of M. walkeri n.
sp. and M. microreticulatus n. sp. might be due to a shift in
pollination syndrome from obligate entomophily to ambophily or anemophily. The
local disappearance (extinction?) of the larger taxa could be related to
climatic changes, which caused the restriction or disappearance of environments
suited for diverse waterside insect populations (including Diptera, diverse
Coleoptera, Heteroptera, etc.: OLSEN et al. 1978). In short, the less derived
members of the Crinopolles Group may have been too specialized by their
dependence for reproduction on the insects responsible for their genetic
isolation and evolution.
Polycolpopollis magnificus n. sp. is the most derived angiosperm-like pollen type in the
Richmond Basin, because its apertures are spiraperturate. Spiraperturate pollen
is found mainly among angiosperms and can be classified under one of two
morphotypes: Pollen with a spiral aperture encircling the grain, and pollen
with rotated apertures on opposites sides of the grain
(FURNESS 1985). P. magnificus n. sp. falls into the second
category. Spiraperturate pollen has functional apertures that involve the
nexine. Some types of polyplicate pollen, such as Equisetosporites chinleana,
resemble spiraperturate pollen, but they have sexinal furrows between their
ridges instead of colpi. Although spiraperturate pollen appears to be
polyphyletic within the angiosperms, its overall association with plants living
in either hot wet or hot dry habitats indicates adaptation to environmental
extremes, and the distribution of its apertures enables germination at numerous
sites, thereby increasing the germination rate (FURNESS 1985). If morphology
and function are related in this species, germination within a fluid-filled
pollen chamber is not indicated for P. magnificus n. sp., because large
pollen size and a reticulate-columellate sculpture imply insect pollination and
germination on a sporophytic stigma. Germination on an integument of a naked
ovule, as in Tsuga and Pinus, would expose the ovules to insect
foraging if insect pollinated, thereby reducing the germination rate. At the
very least, a departure from the gymnospermous mode of germination, the
development of sporophytic self-Incompatibility or SSI (ZAVADA & TAYLOR
1986a), and a shortened reproductive cycle are inferred. P. magnificus
n. sp. was produced by a plant that probably had either a carpel homologue with
stigma or closed cupule with stigma.
The
relevance of reticulate-columellate monosulcate pollen in the Triassic has
already been discussed in the literature since CORNET (1977; 1979; 1980; 1981)
reported angiosperm-like pollen from Late Triassic and Jurassic strata (DOYLE
1978a; 1978b; 1984; NIKLAS et al. 1980; HUGHES 1984; MULLER 1984; HILL &
CRANE 1982; CRANE 1985; DOYLE & DONOGHUE 1986). The full significance of
CORNETs discoveries could not be understood, however, until the data were
effectively published and illustrated here. HILL & CRANE (1982) recognize a
difficulty over the Barremian-Aptian conception of angiosperm origin, because
uncritical acceptance could give rise to circular argumentation: Pre-Barremian
problematic fossils such as Sanmiguelia have come to be regarded as
probable gymnosperms, while post-Barremian problematica have conversely been
accepted as necessarily angiosperms. DOYLE & DONOGHUE (1986) explore
alternative scenarios for the origin of angiosperms, theorizing a possible
Triassic origin of angiosperms after recognizing the sister-group relationship
between the Bennettitales, Gnetales, and Angiospermae. They acknowledge that
all three groups could have had a common origin in the Triassic, but find it
"hard to understand why they [the angiosperms] did not radiate until the
Cretaceous" (DOYLE & DONOGHUE 1986: p. 384). Conversely, CRANE (1985)
finds a Triassic origin followed by low diversity and a delayed radiation an
acceptable hypothesis, when compared to a similar history for the mammals.
Rather than try to force an interpretation for Triassic angiosperm-like pollen
based only on palynological characters, I will summarize pertinent megafossil
data from the Late Triassic Dockum Group of Texas: Well-preserved reproductive
structures, detailed leaf venation and cuticular morphology, and stem, wood,
and root anatomy of Sanmiguelia lewisii have been published and
illustrated by CORNET (1986). Rather than becoming less angiospermous, Sanmiguelia
now appears as a very primitive pre-magnoliid dicot possessing many monocot-like
characters. Although more material needs to be published in order to
substantiate CORNETs interpretations, published data suggest that Sanmiguelia
had a fully developed carpel with apical stigma and closed ventral suture. The
ovuliferous flower was large and unisexual with a differentiated perianth and
an apocarpous gynoecium. Paired sessile anthers were borne by the hundreds on
small male flowers. The anthers were very small, and each possessed a pair of
elliptical pollen sacs adaxially fused to a "conduplicate" bracteole.
The sacs were separated by a septum that disappeared with Pollen maturity, as
in angiosperms. Pollen recovered from the anthers is small, psilate, and
monosulcate with a tectate-granular exine. New but as yet unpublished material
(CORNET 1987, Abs.) discovered since CORNET (1986) confirms the presence of a
pair of anatropous (possibly bitegmic) ovules attached to a fully developed
transmission tissue that flanked the ventral suture. There is also evidence for
insect foraging activity in the form of anther fragments and monosulcate pollen
clinging to the once hairy surface of an isolated carpel (insect jaw marks are
also evident on that carpel compression).
The presence of a possible angiosperm in the Triassic does not necessarily support
the interpretation that crinopolles pollen was produced by early angiosperms,
but it dilutes one major criticism to an angiospermous affinity -
interpretation based only on one organ, with no other supporting data. The fact
that Sanmiguelia produced psilate monosulcate pollen with an
intragranular exine structure is an indication that it is less derived
palynologically than the plants producing crinopolles pollen (cf. WALKER &
SKVARLA 1975). The probable derivation of crinopolles pollen from an auriculate
polyplicate morphotype might suggest that Sanmiguelia and Richmond Basin
angiosperm-like pollen are not closely related. An additional difficulty with
crinopolles pollen being angiospermous is that polyplicate pollen is usually
compared to or assigned to the Gnetales, and cladistic analyses suggest that
the Gnetales are a sister group to the angiosperms, but not the ancestors of
them (CRANE 1985; DOYLE & DONOGHUE 1986; 1987). Thus, the problem focuses
on the basic types of pollen produced by the anthophytes (i. e. the
angiosperms, their ancestors and sister groups), and whether the ancestors of
the angiosperms produced only monosulcate pollen.
The Bennettitales produced mostly small to large psilate monosulcate pollen,
but some taxa (e.g. Williamsoniella lignierii) also produced
inaperturate to indistinctly monoporate pollen (HARRIS 1932; TAYLOR 1973;
FREDERIKSEN 1980; CRANE 1985). The Gnetales, by comparison, produce monosulcate
and inaperturate (holoaperturate) pollen with a broad spectrum of sculpture:
striate monosulcate (e.g. Welwitschia mirabilis: WODEHOUSE 1959; REYRE
1968), polyplicate inaperturate (e.g. Ephedra spp.: ERDTMAN 1965; FOSTER
& GIFFORD 1974; REYRE 1968), granulate inaperturate (e.g. Gnetum indicum:
personal observation; G. africanum: REYRE 1968), echinate inaperturate (G.
leptostachyum: WODEHOUSE 1959), and echinate monosulcate (e.g. Gnetum
sp.: ZAVADA 1984). Monosulcate pollen is considered basic in the Gnetales,
while polyplicate inaperturate pollen is interpreted as derived (DOYLE &
DONOGHUE 1986: p. 421). The fossil record indicates, however, that monosulcate,
polyplicate-inaperturate, and auriculate-polyplicate (Steevesipollenites)
pollen occur together in the Permian (WILSON 1959; CLAPHAM 1970), indicating
that all three were produced by plants living in the same area and possibly
adapted for similar environments. We do not know enough to rule out the
possibility that all three morphotypes may have been produced by closely
related plants, as in the Araceae today.
Dispersed Mesozoic polyplicate pollen can be separated into four distinctive
morphotypes: Welwitschiapollenites (Lagenella) spp.,
Ephedripites (Gnetdceaepollenites) spp., Equisetosporites
spp., and Steevesipollenites spp. Welwitschiapollenites is
striate monosulcate with ribs either parallel or twisted; Ephedripites
is polyplicate inaperturate with ribs oriented parallel to the long axis of the
grain and joined at the poles; Equisetosporites is polyplicate
inaperturate or monosulcate with ribs twisted or rotated on opposite sides of
the grain; and Steevesipollenites is polyplicate inaperturate with
parallel ribs converging on caps or auriculae at the poles. Inaperturate and
monosulcate morphotypes are equally prevalent, and seemingly interchangeable:
If a Welwitschiapollenites, which has parallel ribs, lost its
monosulcus, it would be indistinguishable from Ephedripites, while Lagenella
which has twisted ribs, would resemble Equisetosporites. However, Equisetosporites
chinleana from the same pollen sac possesses either inaperturate or
monosulcate nexinal bodies (ASH 1972: Text-fig. 7, I-J).
I recognize three polyplicate morphotypes in angiosperms: 1) The ephedroid
morphotype with multiple parallel furrows; 2) The equisetosporoid morphotype
with multiple strongly rotated or spiralling furrows; 3) The gnetoid morphotype
with a monosulcate or inaperturate exine (rarely porate), and connate to
spinate sculpture. In some cases the furrows may involve the nexine, making
them apertural or colpate as in Pentecrinopollis traversei n. sp. The
presence or relative abundance of these morphotypes in a particular angiosperm
family is not an indication that the family had a gnetalean ancestry, but
rather an indication of the degree of pollen convergence or parallelism.
Polyplicate and crinopolles morphotypes in angiosperms, if not atavistic, may
provide a comparative level of pollen evolution for pre-tricolpate producing
angiosperms, as well as an indication of what types of pollen were produced by
the ancestors of the angiosperms (cf. POCOCK & VASANTHY 1988). Since the
Gnetales are the only living members of Mesozoic gymnosperms that produced
polyplicate pollen, they may not be representative of the plant that produced Steevesipollenites.
Furthermore, Dechellyia gormanii, which probably produced Equisetosporites
chinleana (ASH 1972), is unlike any extant gnetalean (DOYLE 1978a).
Ephedroid and gnetoid morphotypes in monocots are common in the Araceae (e.g. Spathiphyllum,
and Gonatanthus: THANIKAIMONI 1969; S.
clevelandii produces Steevesipollenites: CORNET, pers.
observation). Gnetoid morphotypes, sometimes with an ulcerate
or porate apertures, also occur in the Alismataceae, Arecaceae (Palmae),
Butomaceae, Dioscoreaceae, Iridaceae, Hanguanaceae, Liliaceae, Najadaceae,
Pandanaceae, Philesiaceae, Xyridaceae, and Zingiberaceae. The more derived
polysulcate morphotypes with compound (pontoperculate) and zonasulculate distal
apertures (cf. Monocrinopollis sp. and Zonacrinopollis sp.) can
be found in the Agavaceae, Arecaceae, Calectasiaceae, Commelinaceae,
Cyclanthaceae, Dasypogonaceae, Dioscoreaceae, Iridaceae, Liliaceae,
Rapateaceae, and Xanthorrhoeaceae (THANIKAIMONI 1970; ALVAREZ & KΦHLER
1987). The trisulcate morphotype (cf. Tricrinopollis sp.) is even found
in the Orchidaceae (i.e. Cypripedium pubescens: HUYNH 1976; Paphiopedilum
faireanum: BURNS-BALOGH 1983, both in the Cypripedioideae). Equisetosporoid
morphotypes in monocots are found in the Aphyllanthaceae, Araceae (e.g.
Holochl~Emys), Eriocaulaceae, Iridaceae, Xanthorrhoeaceae, and Zingiberaceae
(THANIKAIMONI 1970; DAHLGREN et al. 1985). Striate monosulcate pollen (cf. Lagenella
or Welwitschiapollenites) is produced in the Araceae (Pistia
stratiotes), Dioscoreaceae, and Haemodoraceae (THANIKAIMONI 1970).
In the Nymphaeaceae the gnetoid morphotype is common, particularly if spinate
monosulcate pollen is considered. The Nymphaeaceae also produce pollen
resembling crinopolles morphotypes, such as disulcates (e.g. Nymphaea
stellata) and zonasulcates (e.g. Nymphaea d16d: ERDTMAN 1954). The
trisulcate morphotype is a variant produced by Nelumbo (Nelumbonaceae:
KUPRIANOVA 1979).
Connate to spinate pollen resembling that of Gnetum is not as common in
(non-nymphaealean) dicots as it is in monocots. The gnetoid morphotypes can be
found in the Amborellaceae, Aristolochiaceae, Annonaceae, Hernandiaceae,
Lauraceae, and Monimiaceae, all considered primitive dicots (THANIKAIMONI 1970;
WALKER 1974; WALKER 1976a; 1976b). Ephedroid morphotypes, instead of being
found in the more primitive dicot families, are found in such diversified and
advanced families as the Acanthaceae, Polygalaceae, and Polygonaceae. The
equisetosporoid (spiraperturate) pollen type can be found in the Acanthaceae,
Berberidaceae, Bignoniaceae, and Scrophulariaceae (HUANG 1972; BURRMAN 1977;
FURNESS 1985; VASANTHY & POCOCK 1986; POCOCK & VASANTHY 1988).
While the pollen of the Bennettitales is mainly monosulcate (rarely
inaperturate) and psilate, that of the Gnetales is either inaperturate or monosulcate,
and polyplicate, granulate or connate-spinate. The pollen of primitive dicots
and monocots is inaperturate, porate, simple monosulcate, compound monosulcate,
polysulcate, zonasulcate, or spiraperturate, while exine structure may be
polyplicate or atectate-spinate (rarely saccate: ZAVADA & TAYLOR 1986b)
instead of tectate-columellate. The prevalence of pollen morphotypes resembling
either gnetalean pollen or crinopolles pollen suggests that the ancestors of
the angiosperms produced more than monosulcate pollen. A range of alternate
morphotypes that includes some of the forms produced by the Gnetales may be
more realistic than a single morphotype. Gnetoid pollen in angiosperms is
probably derived, as it is in the Gnetales. Crinopolles-like pollen in monocots
is either derived (CHANDA & GHOSH 1976) or atavistic (i.e. a reversion),
leaving simple monosulcate and polyplicate inaperturate pollen as possible
synapomorphies of the angiosperms and Gnetales.
Although the extant
Gnetales do not represent the ancestors of the angiosperms (they have undergone
too much reduction and specialization: DOYLE & DONOGHUE 1987), the
vegetative, anatomical, reproductive, and palynological parallels between these
sister groups suggest a similar but divergent early genetic potential (cf.
VASANTHY & POCOCK 1986; POCOCK & VASANTHY 1988). Since both monosulcate
and polyplicate inaperturate morphotypes can be traced back at least to the
Permian, and the oldest accepted anthophyte fossils are found in the Triassic
(e.g. Bennettitales), the interpretation that reconciles these parallels, while
at the same time accounting for the origin of crinopolles pollen from a
polyplicate, is that the anthophyte ancestors of both groups produced
monosulcate and inaperturate pollen, with polyplicate sculpture being more
common on inaperturate pollen than on monosulcate pollen.
The origin of tricolpate pollen has been a major concern of angiosperm
palynologists, particularly since intermediates between monosulcate and
tricolpate pollen have been difficult to demonstrate. Within the ranalean
complex, only the Nymphaeales produces monosulcate and tricolpate pollen, but
not in the same family (WALKER 1976b): Thirteen of the 35 ranalean families
produce monosulcate pollen, 14 produce inaperturate pollen (five families
produce both morphotypes), and seven produce tricolpate pollen. The
Aristalochiaceae and Chloranthaceae produce a wide range of aperture types,
including anasulcate, inaperturate, polycolpate, polycolpoidate, polyporoidate,
and polyporate pollen, but not tricolpate pollen. In order to explain the
diversity of aperture types, while keeping the monosulcate basic for the
angiosperms, WALKER (1976b) utilizes the inaperturate as an intermediate
between monosulcate and tricolpate pollen. Yet the distribution of ranalean
monosulcate and inaperturate pollen suggests that neither is basic to the other
(cf. NAIR 1979).
At least five possibilities exist for the evolution of tricolpate pollen: The
tricolpate evolved 1) de novo from simple monosulcate pollen as the
Cretaceous pollen record might suggest (DOYLE & HICKEY 1976), 2) de novo
from inaperturate pollen (WALKER 1976b), 3) from trichotomosulcate pollen, 4)
from trisulcate pollen that represents a crinopolles survivor from the Triassic,
or 5) from monosulcate pollen which had passed through a polysulcate stage in
its phylogenetic past. Multiple apertures evolved in separate families within
the ranalean complex (WALKER 1976b), and unless the tricolpate morphotype
evolved only once, an underlying genetic predisposition is implied. A taxon
whose ancestors had gone through a trisulcate or tetrasulcate stage during the
evolution of reticulate-columellate monosulcate pollen might retain suppressed
or modified genes for polysulcate pollen. Although there is no way of
predicting the effects of such genes if they were reactivated, the parent taxon
would have a higher potential for producing polyaperturate pollen (i.e. atavism
or reversal) than a taxon whose ancestors had produced only monosulcate or inaperturate
pollen. Reversals are used in cladistic analyses to explain the presence of a
primitive character in a derived lineage whose immediate ancestor(s) did not
possess it (cf. DOYLE & DONOGHUE 1986). The selective pressures responsible
for rapid pollen evolution in the Aptian and Albian may have unlocked a core of
dormant genes involved in earlier pollen experimentation or variability.
The Nymphaeales are regarded by some botanists as a group intermediate between
the monocots and dicots, and are usually placed on phylogenetic trees either
below the monocots (pre-monocot: WALKER & WALKER 1984) or below magnoliid
dicots (HICKEY & WOLFE 1975). DAHLGREN et al. (1985) consider the
Nymphaeales, along with the Piperales and Annonales, as the closest living
dicot orders to the more primitive monocot orders, the Dioscoreales, Arales,
and Alismatales. The Crinopolles Group, with its diversity of apertures and
monocot-like exine structure and sculpture, also falls in the morphological gap
between monocots and dicots. Nelumbo pollen ranges from tricolpate to
trisulcate with a pair of equatorial sulci positioned as in Tricrinopollis
n. gen. (KUPRIANOVA 1979). Kuprianova regards the trisulcate condition in
extant Nebmbo pollen as a vestige of the palynological transition from
monosulcate to tricolpate dicot pollen. Tetrad orientation ranges from radial
to bilateral (KUPRIANOVA 1979) as in T. olsenii n. sp. The equatorial
sulci occasionally join to form a ring sulcus, as in Zonacrinopollis
anasulcatus n. sp. These similarities between Nelumbo pollen and Tricrinopollis
spp. raise the possibility that the polysulcate morphotype may have reappeared
in the Nelumbonaceae (atavism), allowing this family to depart from the typical
monosulcate morphotype of other water lilies. Similarly, tetrasulcate pollen in
Calectasia cyanea (extant Calectasiaceae) may have secondarily evolved
because of a genetic predisposition for multiple distal apertures, even though
it probably evolved from monosulcate pollen (CHANDA et al. 1978).
In the mid-Cretaceous when the dicots were undergoing rapid diversification and
migration, the climatic and edaphic changes that were supporting this radiation
may have also benefitted the immediate ancestors of the angiosperms. Within
semiarid tropical paleolatitudes, various exotic polyplicate morphotypes
evolved during a major Early Cretaceous radiation of polyplicate pollen (DOYLE
& DONOGHUE 1987: p. 178), and are recorded under such names as Galeacornea
spp., Elaterosporites spp., EIaterocolpites spp., EIateroplicites
spp., Pentapsis spp., Senegalosporites spp., and Sofrepites
spp. JARDINΙ 1967). Some of these types appear to be derived from Steevesipollenites
spp., which is also present, while others appear to have evolved similar
aperture morphology as Tricrinopollis and Zonacrinopollis (e. g. Galeacornea
clavis STOVER 1963). This radiation of polyplicate morphotypes at a time
when angiosperms were creating new floral communities and alliances, suggests
that the plants producing them were closely enough related to angiosperms that
they were able to "ride on the coat tails" of the angiosperm success,
and took advantage of the insect vectors available to the angiosperms at that
time The parallel radiation of angiosperm and polyplicate pollen in the
mid-Cretaceous would have little significance other than an added dimension to
the parallels between angiosperms and the Gnetales without the knowledge of
possible morphological and evolutionary relationships between angiosperm-like
pollen and Steevesipollenites in the Triassic.
A record of pre-Cretaceous angiosperm-like pollen is now taking form through
persistent searching for rare occurrences of pollen that might otherwise be
disregarded or dismissed as contamination. This record is still very
incomplete, but is sufficient to show that much more was happening in the
Triassic than previously believed. If pollen with
reticulate-columellate-footlayer ectexinal and non-laminated endexinal
structure proves to be an angiosperm autapomorphy as DOYLE & DONOGHUE
(1986) contend (and therefore unique to angiosperms), then the Triassic
Crinopolles Group probably evolved within the angiosperms rather than within a
pre-angiospermous anthophyte group, and the earliest angiosperms may have
produced polyplicate inaperturate as well as tectate-granular monosulcate
pollen. The presence of possible angiosperm megafossils in the Dockum Group of
Texas and in the Richmond Basin of Virginia (CORNET 1986) warns against
premature conclusions that Early Cretaceous angiosperms are early angiosperms,
or that a poor pre-Cretaceous fossil record for angiosperms has phylogenetic
significance with regard to the time of angiosperm origin. Megafossil and pollen
data, together with the results of cladistic analyses, suggest that the
Jurassic may not be as devoid of angiosperms as conventional interpretations of
Jurassic psilate monosulcate and polyplicate pollen might indicate (cf. DOYLE
1978a; POCOCK & VASANTHY 1988). Many of the events in the evolution of the
angiosperms are still unclear, but a new chapter has been added that highlights
the Triassic as the Period to search for evidence of the earliest phases of
angiosperm evolution.
The estimated cost of duplicating the data presented in this paper, taking into
consideration the inclusive costs of drilling two wells for cuttings and
correlation purposes (96%), field work expenses in collecting outcrop samples,
sample processing costs in the laboratory, microscope time for picking,
mounting, and studying individual grains and for working the well samples at
oil company rates, photographic costs leading up to the selection of
illustrations for plates, and the man-hours required to write this manuscript,
is about $1,250,000.
I wish to
acknowledge the help and assistance of The Pennsylvania State University, the
National Science Foundation (grant no. GA36870 to Professor ALFRED TRAVERSE),
the late Gulf Oil Corp. - Houston, Exxon Co. U.S.A. - Houston, the late
Geminoil Inc. Houston, Cornell Oil Company - Dallas, the late Superior Oil
Company - Houston, and the University of Houston for providing the facilities,
equipment, and financial support needed to make this study possible. I also wish
to acknowledge the following people: Dr. WILLIAM C. BURGER, the late GEORGE R.
FOURNIER, Prof. PAUL E. OLSEN, Dr. ELEANORA I. ROBBINS, Dr. LEWIS E. STOVER,
the late Dr. GANAPATHI THANIKAIMONI, Prof ALFRED TRAVERSE, and GERALD P WILKES
for providing data or reference material; Prof VAUGHN M. BRYANT, Prof. ARTHUR
V. CHADWICK, ROBERT R. KEITH, THOMAS D. SHELL, AUDREY G. WALKER, and Prof.
JAMES W.WALKER for providing SEM and TEM micrographs of specimens; and Prof.
BRUCE K. GOODWIN, Prof. PAUL E. OLSEN, and ROBERT E. WEEMS for field assistance
and geologic interpretations. I wish to additionally thank the WALKERS for
their excellent quality SEM and TEM work, G. R. FOURNIER and Dr. P E. OLSEN for
many hours of helpful discussions and advice, and my wife, BONNIE LEE CORNET,
for her support and encouragement during the final stages of completing this
manuscript. The well data presented in this paper belong to the author, who was
the President of Geminoil, Inc., the company behind
the drilling of the Horner and Bailey wells.
ALVAREZ, A. &
KΦHLER, E. (1987): Morfologia del polen de las Agavaceae y algunos generos
afines. - Grana, 26: 25-46.
ASH, S. R. (1972):
Late Triassic plants from the Chinle Formation in north-eastern Arizona. -
Palaeont., 15: 598-618.
BALME, B. E.
(1970): Palynology of Permian and Triassic strata in the Salt Range and Surghar
Range, west Pakistan. - In KUMMEL, B. & TEICHERT,
C. (eds.): Stratigraphic boundary problems: Permian and Triassic of west Pakistan. - Univ. of Kansas Spec. Publ. 4: 305-453.
BOCK, W. (1969):
The American Triassic flora and global distribution. - Geol. Cntr. Res. Ser.,
23, 406 p. - North Wales, PA.
BAKKER, R. T
(1978): Dinosaur feeding behavior and the origin of flowering plants. - Nature,
274: 661-3.
BINO, R. J., DAFNI,
A. & MEEUSE, A. D. J. (1984): Entomophily in the dioecious gymnosperm
Ephedra dphylla Forsk. (= E. dlte C. A. Mey.), with some notes on E.
campylopoda C. A. Mey. I. Aspects of the entomophilous syndrome. - Proc.
Koninklijke Nederlandse Akademie van Wetenschappen, Series C 87: 113.
BRENNER, G. J.
(1987): Paleotropical evolution of the Magnoliidae in the Lower Cretaceous of
Northern Gondwana. - Bet. Sec. Amer. Abstrs., 74: 677-8.
BRUGMAN, W. A.
(1983): Permian-Triassic palynology. - Lab. Palaeob. Palyn.,
State Univ. Utrecht, 121 p. - Heidelberglaan, The Netherlands.
BURNS-BALOGH, P
(1983): A theory on the evolution of the exine in Orchidaceae. - Amer. J. Bet.,
70: 1304-12.
SUURMAN, J. (1977):
Contribution to the pollenmorphology of the Bignoniaceae, with special
reference to the tricolpate type. - Pollen et Spores,
19: 447-519.
CHANDA, S. &
GHOSH, K. (1976): Pollen morphology and its evolutionary significance in
Xanthorrhoeaceae. - In FERGVSON, I. K. & MULLER, T.
(eds.): The evolutionary significance of the exine. - Linean Sec. Symp. Ser.,
1: 527-59. - Academic Press Inc., New York.
CHANDA, S.,
LUGARDON, B. & THANIKAIMONI, G. (1978): On the ultrastructure of pollen
aperture in Calectasia R. Br. (Xanthorrhoeaceae). - Pollen et
Spores, 20: 351-65.
CLAPHAM JR., W B.
(1970): Permian miospores from the Flowerpot Formation of western Oklahoma. -
Micropaleont., 16: 15-36.
CORNET, B. (1977):
The palynostratigraphy and age of the Newark Supergroup. unpublished
Ph. D. thesis, The Pennsylvania State University, 505 p.
-,-
(1979): Angiosperm-like pollen with tectate-columellate wall structure from the
Upper Triassic (and Jurassic) of the Newark Supergroup, U.S.A. (Abs.). Palynol., 3: 281-2.
-,-
(1980): Tropical Late Triassic monosulcate and polysulcate angiospermid pollen
and their morphological relationship with associated auriculate polyplicate
pollen (Abs.). 5ch Intern. Palyn. Conf., Cambridge (1980): 91.
-,-
(1981): Recognition of pre-Cretaceous angiosperm pollen and its relationship to
fossil polyplicate pollen (Abs.). - Palynology, 5: 212-3.
-,-
(1986): The reproductive structures and leaf venation of a Late Triassic
angiosperm, Sanmiguelia
lewisii. - Evol. Theory, 7: 231-309.
-,-
(1987): Further evidence for the reproductive morphology of Sanmiguelia
lewisii and its bearing on angiosperm ancestry (Abs. 237). - Amer. J.
Bot. 74: 680.
CORNET, B. &
OLSEN, P. E. (1985): A summary of the biostratigraphy of the Newark Supergroup
of eastern North America with comments on Early Mesozoic provinciality. - In
Simposio Sobre Floras del Triassico Tardio, su Fitogeografia y Paleoecologia.
Memoria, 67-81. - III Congresso Latinoamericano de Paleontologia. Mexico.
CORNET, B. &
TRAVERSE, A. (1975): Palynological contributions to the chronology and
stratigraphy of the Hartford Basin in Connecticut and Massachusetts. -
Geosci. and Man, 11: 1-33.
CORNET, B. &
ZIEGLER, D. G. (1985): Structural styles and tectonic implications of
Richmond-Taylorsville Rift System, Eastern Virginia (Abs.). - A.A.P.G.
Bull., 69: 1434.
CRANE, P. R.
(1985): Phylogenetic analysis of seed plants and the origin of angiosperms. -
Ann. Missouri Bot. Gard., 72: 716-93.
-,-
(1986): Form and function in wind dispersed pollen. - In BLACKMORE, S. &
FERGUSON, I. K. (eds.): Pollen and spores: form and function. - Linn. Soc.
London: 179-202. - Academic Press, London.
DANZΙ-CORSIN, P.
& LAVEINE, J.-P. (1963): Microflore. - In BRICHE, P., DANZΙ-CORSIN, P.
& LAVEINE, J.-P.: Flore Infraliasique du Boulonnais (macro- et microflore). - Societe Geologique du Nord, Mem., 13 (B):
57-135.
DAHLGREN, R. M. T.,
CLIFFORD, H. T. & YEO, P. F. (1985): The families of the monocotyledons,
structure, evolution, and taxonomy. - Springer- Verlag, New York, 520 p.
DAUGHERTY, L. H.
(1941): The Upper Triassic flora of Arizona, with a discussion of its
geological occurrence. - Contrib. Paleont., Carnegie Inst., Washington,
526: 108 p.
DELEVORYAS, T.
(1970): Plant life in the Triassic of North Carolina. - Discovery, 6: 15-22.
DELEVORYAS, T.
& HOPE, R. C. (1973): Fertile coniferophyte remains from the Late Triassic
Deep River Basin, North Carolina. - Amer. J. Bot., 60: 810-8.
-,- (1975): Voltzia
andrewsii, n. sp., an Upper Triassic seed cone from North Carolina, U.S.A.
- Rev. Palaeo bot. Palynol., 20: 67-74.
-,-
(1981): More evidence for conifer diversity in the Upper Triassic of North
Carolina. - Amer. J. Bot., 68: 1003-7.
DOYLE, J. A. (1973): Fossil evidence on early evolution of the
monocotyledons, in The monocotyledons: their evolution and comparative biology.
- Quart. Rev. Bioi., 48: 399-413.
-,-
(1977): Patterns of evolution in early angiosperms. - In HALLAM, A. (ed.):
Patterns of evolution. - Elsevier Sci. Pub I. Co., 501-46. - Amsterdam.
-,-
(1978a): Origin of angiosperms. - Ann. Rev. Ecol. Syst., 9: 365-92.
-,-
(1978b): Potentials and limitations of exine structure in studies of Early
Cretaceous angiosperm evolution. - Cour. Forsch.-Inst. Senckenberg, 30:
54-61. - Frankfurt am Main.
DOYLE, J. A.,
BIENS, P., DOERENKAMP, A. & JARDINE, S. (1977): Angiosperm pollen from the
pre-Albian Lower Cretaceous of equatorial Africa. - Bull. Rech.
Explor-prod. Elf-Aquitaine, 1 (2): 451-73.
DOYLE, J. A. &
DONOGHUE, M. J. (1986): Seed plant phylogeny and the origin of angiosperms: An
experimental cladistic approach. - Bot. Rev., 52: 321-431.
-,-
(1987): Relationships of angiosperms and Gnetales: a numerical cladistic
analysis. - In SPICER, R. A. & THOMAS, B. A. (eds.): Systematic and
taxonomic approaches in paleobotany. - Syst. Assoc. Spec. Vol. 31: 177-98. -
Clarendon Press, Oxford.
DOYLE, J. A. &
HICKEY, L. J. (1976): Pollen and leaves from the mid-Cretaceous Potomac Group
and their bearing on early angiosperm evolution. - In BECK, C. B. (ed.): Origin
and early evolution of angiosperms, 139-206. - Columbia University Press, New
York.
DOYLE, J. A., VAN
CAMPO, M. & LUGARDON, B. (1975): Observations on
exine structure of Eucommiidites and Lower Cretaceous angiosperm pollen.
- Pollen et Spores, 17: 430-86.
DUNAY, R. E. &
FISHER, M. J. (1974): Late Triassic palynofloras of North America and their
European correlatives. - Rev. Palaeobot. Palynol., 17:
179-86.
-,- (1979): Palynology of the Dockum Group (Upper Triassic),
Texas, U.S.A. - Rev. Palaeobot. Palynol., 28: 61-92.
ERDTMAN, G.
(1954): An inttoduction to pollen analysis. - Chronica Botanica Co., 239 p. -
Waltham, Mass.
-,- (1965): Pollen and spore morphology/plant taxonomy.
Gymnospermae, Bryophyta (text). (An introduction to palynology. III). -
Almqvist & Wiksell, 191 p. - Stockholm.
EDIGER, V. S. (1986):
Paleopalynological biostratigraphy, organic matter deposition, and basin
analyses of the Triassic-Jurassic(?) Richmond
rift basin, VA. - unpublished Ph. D. thesis, The
Pennsylvania State University, 547 p.
FISHER, M. J. &
DUNAY, R. E. (1984): Palynology of the Petrified Forest Member of the Chinle
Formation (Upper Triassic), Arizona, U.S.A.- Pollen et
Spores, 26: 241-83.
FURNESS, C. A.
(1985): A review of spiraperturate pollen. - Pollen et
Spores, 27: 307-20.
FREDERIKSEN, N. O.
(1980): Significance of monosulcate pollen abundance in Mesozoic sediments. -
Lethaia: 13: 1-20.
FREDERIKSEN, N. 0.,
WIGGINS, V. D., FERGUSON, I. K., DRANSFIELD, J. & AGER, C. M. (1985):
Disttibution, paleoecology, paleoclimatology, and botanical affinity of
the Eocene pollen genus Diporoconia n. gen. - Palynology, 9: 37-60.
GALLOWAY, W. E.
(1968): Depositonal systems of the Lower Wilcox Group, north-central Gulf Coast
Basin. - Trans. Gulf Coast Assoc. Geol. Soc., 28: 275-89.
GOODWIN, B. K.,
WEEMS, R. E., WILKES, G. P., FROELICH, A. J. & SMOOT, J. P. (1985): The
geology of the Richmond and Taylorsville basins, east-centtal Virginia. - E.
Sect. A.A.P.G. Meeting Field Trip 4 Guidebook, 60 p.
HARRIS, T. M.
(1932): The fossil flora of Scoresby Sound East Greenland. 3: Caytoniales and
Bennettitales. - Medd. Groenl., 85 (5): 133 p.
HERNGREEN, G. F. W.
(1974): Middle Cretaceous palynomorphs from northeastern Brazil. - Sci. Geol.,
Bull., 27: 101-16.
HICKEY, L. J. &
DOYLE, J. A. (1977): Early Cretaceous fossil evidence for angiosperm evolution.
- Bot. Rev. (Lancaster), 43: 3-104.
HICKEY, L. J. &
WOLFE, J. A. (1975): The bases of angiosperm phylogeny: vegetative morphology.
- Ann. Missouri Bot. Gard., 62: 538-89.
HILL, C. R. &
CRANE, P. R. (1982): Evolutionary cladistics and the origin of angiosperms. -
In JOYSEY, K. A. & FRIDAY, A. E. (eds.): Problems of phylogenetic
reconstruction. - Systematics Assoc. Spec. Vol. 21: 269-361. - Academic Press,
London and New York.
HOPE, R. C. &
PATTERSON III, O. F. (1969): Triassic flora from the Deep River Basin, North
Carolina. - N. C. Dept. Conserv. and Develop., Div.
Min. Res., Spec. Pub I. 2: 23 p.
HOROWITZ, A.
(1970): Jurassic microflora from the northern Negev, Israel. - Israel J.
Earth-Sci., 19: 153-82.
HUANG, T.-G.
(1972): Pollen flora of Taiwan. - Nation Taiwan Univ. Bot. Dept. Press, 297 p.
- Taiwan.
HUGHES, N. F.
(1984): Mesosperm palynologic evidence and ancestors of angiosperms. - Ann.
Missouri Bot. Gard., 71: 593-8. HUYNH, K.-L. (1976): Quelques phenomenes de
polarite du pollen des Orchidaceae. - Ber. Schweiz. Bot. Ges., 86 (3-4):
115-28.
JAIN, R. K. (1968):
Middle Triassic pollen grains and spores from Minas de Petroleo beds of the
Cacheuta Formation (Upper Gondwana), Argentina. - Palaeontographica, 122
B: 1-47.
JARDINE, S. (1967):
Spores a expansions en forme d'elateres du Cretace
Moyen d'Afrique occidentale. - Rev. Palaeobot. Palynol.,
1: 235-58.
KEDVES, M. J.
(1985): Structural modification of degraded fossil sporomorphs. - Micropaleont.,
31: 175-80.
KLAUS, W. (1979):
Zur entwicklungsgeschichtlichen Bedeutung triadischer, angiospermider
Pollenapertur- und Strukturanlagen. - Beitr. Palaeontol. Osterr.,
6: 135-77.
KOOB, J. D. (1961):
Triassic pollen and spore flora of the Cumnock Formation (Newark Series) of
North America. - unpubl. Master of Arts thesis,
University of Massachusetts, 67 p.
KUPRIANOVA,
L. A. (1979): On the possibility of the development of tricolpate pollen from
monosulcate. - Grana, 18: 1-4.
LAWAL, O. &
MOULLADE, M. (1987): Palynological biostratigraphy of Cretaceous sediments in
the upper Benue Basin, N.E. Nigeria. - Rev. Micropaleont., 29: 61-83.
LE THOMAS, A. &
LUGARDON, B. (1975): Ultrastructure d'un pollen original parmi les Annonacees.
- In Soc. bot. Fr., Coli. Palynologie: 109-11.
LITWIN, R. J.
(1985): Fertile organs and in situ spores of ferns from the Late Triassic
Chinle Formation of Arizona and New Mexico, with discussion of the
associated dispersed spores. - Rev. Palaeo bot. Palynol.,
44: 101-46.
LUND,J. J. (1977): Rhaetic to Lower Liassic palynology of the
onshore south-eastern North Sea Basin. - Geol. Surv. Denmark, Series II (109):
129 p.
MULLER, J. (1984):
Significance of fossil pollen for angiosperm history. - Ann. Missouri Bot.
Gard., 71: 419-43.
NAIR, P. K. K.
(1979): The palynological basis for the triphyletic theory of angiosperms. -
Grana, 18: 141-4.
NIKLAS, K. J.,
TIFFNEY, B. H. & KNOLL, A. H. (1980): Apparent changes in the diversity of
fossil plants. - In HECHT, M. K., STEERE, W. C. & WALLACE, B.
(eds.): Evolutionary Biology, 12: 1-89. - Plenum Pub. Corp., New York.
OLSEN, P. E.
(1986): A 40-million-year record of Early Mesozoic orbital climatic forcing. -
Science, 234: 789-848.
OLSEN, P. E.,
REMINGTON, C. L., CORNET, B. & THOMSON, K. S. (1978): Cyclic change in Late
Triassic lacustrine communities. - Science, 201: 729-33.
POCOCK, S. A. J.
(1978): Lowermost Jurassic spore-pollen assemblage from Canadian Arctic. - The
Palaeobotanist, 25: 363-75.
POCOCK, S. A. J.
& VASANTHY, G. (1988): Cornetipollis reticulata, a new pollen with
angiospermid features from Upper Triassic (Carnian) sediments of Arizona
(U.S.A.), with notes on Equisetosporites. - Rev. Paleobot. Palynol., 55: 337-56.
PRAGLOWSKI, J.
(1974): Magnoliaceae Juss. - In NILSSON, S. (ed.): World pollen and spore flora
3. - Almqvist & Wiksell Period. Co.: 1-44. - Stockholm.
RETALLACK, G. &
DILCHER, D. L. (1981): A coastal hypothesis for the dispersal and rise to
dominance of flowering plants. - In NIKLAS, K. J. (ed.): Palaeobotany,
Paleoecology and Evolution, 27-77. - Praeger Publishers, New York.
RETALLACK, G. J.
& DILCHER, D. L. (1986): Cretaceous angiosperm invasion of North America. -
Cretaceous Research, 7: 227-52. - Academic Press Inc. Ltd., London.
REYRE, Y. (1968):
La sculpture de l'exine des pollens des gymnospermes et
des chlamydospermes et son utilisation dans l'identification des pollens
fossiles. - Pollen et Spores, 10: 197-220.
-,-
(1973): Palynologie du Mesozoique Saharien. - Mem. Mus. Nation. d'Hist. Natur., Serie C, 27: 284.
ROBBINS, E. I.
(1982): "Fossil Lake Danville": The paleoecology of a Late Triassic
ecosystem on the North Carolina-Virginia border.-
unpublished Ph. D. thesis, The Pennsylvania State Univ., 383 p.
ROWLEY, J. R. &
SOUTHWORTH, D. (1967): Deposition of sporopollenin on lamellae of unit membrane
dimensions. - Nature, 213: 703-4.
ROWLEY, J. R. &
SRIVASTAVA, S. K. (1986): Fine structure of Classopollis exines. - Can.
J. Bot., 64: 3059-74.
SCHAEFFER, B. &
McDONALD, N. G. (1978): Redfieldiid fishes from the Triassic-Liassic Newark
Supergroup of eastern North America. - BulL Amer. Mus. Nat. Hist., 159: 129-74.
SCHULTZ, E. (1967):
Sporenpalaontologische Untersuchungen ratoliassischer Schichten im Zentralteil
des Germanischen Beckens: Palaont~. logische
Abhandlungen, Abt. B (2): 544-633.
SCHULTZ, G. &
HOPE, R. C. (1973): Late Triassic microfossil flora from the Deep River Basin,
North Carolina. - Palaeont. Abt. B, 141: 63-88. SCOTT, R. A. (1960): Pollen of Ephedra
from the Chinle Formation (Upper Triassic) and the genus Equisetosporites.
- Micropaleont., 6: 271-6.
SHALER, N. S. &
WOODWORTH, J. B. (1899): Geology of the Richmond Basin, Virginia. - U.S.G.S.
19th Ann. Rept., 2: 385-515.
STONE, J. F.
(1978): Pollen and Spores. - In Ash, S. (ed.): Geology, Paleontology, and Paleoecology
of a Late Triassic lake, Western New Mexico. - Brigham Young Univ. Geol.
Studies, 25 (2): 45-59.
STOVER, L. E.
(1964): Cretaceous ephedroid pollen from West Africa. - Micropaleont., 10:
145-156.
TAYLOR, T. N.
(1973): A consideration of the morphology, ultrastructure and multicellular
microgametophyte of Cycadeoidea dacotensis pollen. - Rev. Palaeo
bot. Palyn., 16: 157-164.
TAYLOR, T. N. &
ALVIN, K. L. (1984): Ultrastructure and development of Mesozoic pollen:
Classopollis. - Amer. J. Bot., 71: 575-81;
TAYLOR, T. N.,
ZAVADA, M. S. & ARCHANGELSKY, S. (1987): The ultrastructure of Cyclusphaera
psilata from the Cretaceous of Argentina. - Grana, 26: 74-80.
THANlKAIMONI, G.
(1969): Esquisse palynologique des Aracees. - Tr. Sect. Sci. Tech., Inst. Fr.
Pondichery, 5 (5): 29 p.
-,-
(1970): Les palmiers: palynologie et systematique. - Tr. Sect. Sci. Tech.,
Inst. Fr. Pondichery, 11: 1-286.
TRAVERSE, A.
(1986): Palyno.logy of the Deep River Basin, North Carolina. - In Gore, P.J.W.
(ed.): Depositional framework of a Triassic rift basin: The Durham and Sanford
sub-basins of the Deep River Basin, North Carolina. - Soc. Econ. Paleont. Min.
Meeting Field Trip 3 Guidebook, 66-71.
TREVISAN, L.
(1980): Ultrastructural notes and considerations on Ephedripites,
Eucommiidites, and Monosulcites pollen grains from Lower Cretaceous
sediments of southern Tuscany (Italy). - Pollen et
Spores, 22: 85-132.
VASANTHY, G. &
POCOCK, S. A. J. (1986): Radia.l through rotated symmetry of striate pollen of
the Acanthaceae. - Can. J. Bot., 64: 3050~8.
VOLKHEIMER, W.
& ZAVATTIERI, A. M. (1985): Una microflora Triasica de la localidad de
divisadero large (Mendoza, Argentina). - In Simposio Sobre Floras del
Triassico Tardio, su Fitogeografia y Paleoecologia. Memoria, 43-51. - III Congresso
Latinoamericano de Paleontologia. Mexico.
WAHA, M. (1987):
Sporoderm development of pollen tetrads in Asinima triloba (Annonaceae).
- Pollen et Spores, 29: 31-44.
WALKER, J. W.
(1974): Aperture evolution in the pol1en of primitive angiosperms. - Amer. J.
Bot., 61: 1112-37.
-,-
(1976a): Evolutionary significance of the exine in the pollen of primitive
angiosperms. - In FERGUSON, I. K. & MULLER, J. (eds.): The evolutionary
significance of the exine. - Linean Soc, Symp. Ser., 1: 251-308. - Academic
Press Inc., New York.
-,-
(1976b): Comparative pollen morphology and phylogeny of the ranalean complex. -
In BECK, C. B. (ed.): The origin and early evolution of angiosperms, 241-299. -
Columbia Univ. Press, New York.
WALKER, J. W.,
BRENNER, G. J. & WALKER, A. G. (1983):
Winteraceous pollen in the Lower Cretaceous of Israel: early evidence of a
magnolia.lean angiosperm family. - Science, 226: 1273-5.
WALKER, J. W. &
SKVARLA, J. J. (1975): Primitively columellaless pollen: a new concept in the
evolutionary morphology of angiosperms. - Science, 187: 445-7.
WALKER, J. W. &
WALKER, A. G. (1984): Ultrastructure of Lower Cretaceous angiosperm pollen and
the origin and early evolution of flowering plants. - Ann. Missouri Bot. Gard.,
71: 464-521.
WARD, J. V. (1986):
Early Cretaceous angiosperm polten from the Cheyenne and Kiowa formations
(Albian) of Kansas, U.S.A. - Palaeont. Abt. B, 202: 1-81.
WEEMS, R. E.
(1980a): An unusual newly discovered archosaur from the Upper Triassic of
Virginia, U.S.A. - Trans. Amer. Phil. Soc., 70: 1-53.
-,-
(1980b): Geology of the Taylorsville Basin, Hanover County, Virginia. -
Contrib. Virgo Geol. - IV, 27: 23-38.
WILSON, L. R.
(1959): Geological history of the Gnetaks. - Oklahoma Geol. Notes, 19: 35-9. -
Ok. Geol. Surv., Norman.
WODEHOUSE, R. P.
(1959): Pollen grains. Their structure, identification and significance in
science and medicine. - Hafner Publ. Co. - New York.
ZAVADA, M. S.
(1984): Angiosperm origins and evolution based on dispersed fossil pollen
untrastructure. - Ann. Missouri B~t. Gard., 71:
444-63.
ZAVADA, M. S. &
DILCHER, D. L. (1988): Pollen wall untrastructure of selected dispersed
monosulcate pollen from the Cenomanian, Dakota Formation, of central USA. -
Amer. J. Bot., 75: 669-79.
ZAVADA, M. S.
& TAYLOR, T. N. (1986a): The role of self;.incompatibility
and sexual selection in the gymnosperm-angIosperm transition:
A hypothesis. - Amer. Nat., 128: 53&-49.
-,- (1986b): Pollen morphology of Lactoridaceae. - Pl. Syst.
Evol., 154: 31-9.
All figures X1000
unless otherwise indicated *
Plate 1
Figs 1 - 9. Pentecrinopollis
traversei n. sp.
1 - 2. Holotype (81 X 55 microns); SGM-PI. 1. Distal focus. 2. Proximal focus. 3. (74 X 45 microns) distal focus. 4 - 5, 8. Same grain (*86 X 50 microns). 4. SEM enlargement, proximal side. 5. SEM, lateral view. 6. SEM (*77 X 51 microns), lateral view. 7, 9. Entire specimen not shown (88 X 50 microns). 7. SEM enlargement of distal ridge. 8. SEM enlargetnent of a distal sulcus. 9. SEM enlargement showing reticulum. Scale in microns for Figs. 4, 79. SEM Inicrographs by J. W. WAI.KER.
Figs. 10 - 13. Pentecrinopollis
gemmatus n. sp.
10. (38 X 25 microns) Lateral compression; note enlarged apical gemmae. 11. (36 X 28 microns) Lateral compression, proximal side at bottom. 12 - 13. Holotype (32 X 20 microns), lateral compression; SGM-Q1. 12. Low focus, proximal side at bottom. 13. High focus; note protruding proximal patch.
Plate 2
Figs. 14 - 24. Tricrinopollis
olsenii n. sp.
14 - 16. Tetrahedral tetrad (individual grains 42-45 microns long; 25-30 microns wide). 14. Low focus. 15. Median focus. 16. High focus; note upper grain bent at right angle. 17 - 18. Holotype (48 X 30 microns), oblique-lateral compression; SGM-I21. 17. Distal side in focus, with median sulcus and a lateral sulcus superimposed. 18. Proximal side in focus. 19-20, 23 - 24. Same grain (62 X 38 microns). 19. SEM, oblique distal view showing median sulcus and one of two lateral sulci. 20. TLM, proximal side and second lateral sulcus in focus. 21 - 22. Same grain (46 X 30 microns). 21. SEM, oblique proximal view showing one of two lateral sulci. 22. TLM, distal side with median sulcus and second lateral sulcus in focus. 23. SEM enlargement of fine reticulum on distal side. 24. SEM enlargement of lateral side showing transition from fine to coarse reticulum. SEM micrographs by J. W. WALKER.
Plate 3
Figs. 25 - 35. Monocrinopollis
doylei n. sp. (also Figs. 53 - 56).
25 - 26. (41 X 36 microns). 25. Proximal-distal compression with distal side in focus. 26. Proximal side in focus. 27. (40 X 39 microns), distal side in focus; note sub-rounded aperture. 28, 32. Same grain (39 X 33 microns). 28. TLM, proximal side in focus. 29, 30. Same grain (42 X 33 microns). 29. TLM, proximal-distal compression with proximal side in focus. 30. SEM, distai view showing a short anomalous sulcus to the right of the main aperture. 31. SEM (44 X 35 microns), oblique lateral view showing transition from fine to coarse sculpture. 32. SEM (39 X 33 microns), distal view showing trichotomosulcate aperture. 33, 34. Same grain (48 X 35 microns). 33. SEM enlargement of proximal reticulum supported by columellae. 34. SEM, oblique lateral view showing transition from coarse to fine sculpture. 35. SEM (45 X 34 microns), lateral view. SEM micrographs by J. W. WALKER.
Figs. 36 - 41. Tricrinopollis
minutus n. sp.
36 - 37. Holotype (30 X 20 microns); SGM-J1. 36. Oblique lateral compression with distal side in focus. 37. Proximal side in focus. 38-39. Same grain (37 X 25 microns). 38. Proximal-distal compression with proximal side in focus. 39. Distal side in focus; note an anomalous aperture to the right of the median distal sulcus, making this grain tetrasulcate. 40. (36 X 25 microns) Oblique proximal-distal compression with proximal side in focus; three sulci are visible. 41. Rare diad (32 X 27; 31 X 24 microns); proximal-distal compressions of a pair probably attached in their relative tetrad positions.
Plate 4
Figs. 42 - 52. Monocrinopollis
mulleri n. sp.
42 - 43. Holotype (45 X 33 microns); SGM-C3. 42. Proximal-distal compression with proximal side in focus. 43. Distal side in focus. 44. (44 X 33 microns) Proximal-distal compression with distal side in focus. 45. (36 X 28 microns) Proximal-distal compression with proximal side in focus. 46, 50. Same grain (43 X 30 microns). 46. SEM, proximal view. 47. SEM (43 X 27 microns), oblique distal view showing transition in sculpture. 48, 51. Same grain (45 X 29 microns). 48. SEM, oblique proximal view showing a short anomalous sulcus on lateral side in same position as in Tricrinopollis. 49. SEM (45 X 37 microns), distal view showing a three-sided trichotomosulcoid aperture. 50. SEM enlargement of proximal reticulum supported by columellae. 51. SEM enlargement of proximal reticulum supported by columellae. 52. (45 X 37 microns). Unoxidized specimen showing a dark nexine beneath a clear sexine. SEM micrographs by J. W WALKER & R. R. KEITH.
Figs. 53 - 56. Monocrinopollis
doylei n. sp.
53. Holotype (45 X 35 microns), proximal-distal compression with the distal side in focus in the upper part of the grain, and the proximal side in focus in the lower part; SGM-A22. 54. (47 X 36 microns) Proximal-distal compression with mainly the proximal side in focus. 55 - 56. Same grain (50 X 36 microns). 55. Proximal-distal compression with distal side in focus. 56. Proximal side in focus.
Plate 5
Figs. 57 - 62. Dicrinopollis
operculatus n. sp.
57. Holotype (42 X 30 microns), Oblique lateral compression with distal side in focus; SGM-L2. 58-60. Same grain (46 X 32 microns). 58. SEM, oblique proximal-dorsal view showing proximal sculpture. 59. TLM, proximal side in focus. 60. TLM, distal side in focus; note large detached operculum formed by widely-spaced sulci. 61. (45 X 33 microns) Oblique lateral compression with distal side in focus; note broken operculum. 62. SEM (38 X 25 microns), distal view showing operculum. SEM micrographs by R. R. KEITH.
Figs. 63 - 66. Monocrinopollis
microreticulatus n. sp.
63, 65 - 66. Same grain (31 X 25 microns). 63. TLM, proximal-distal compression with Portions of both sides in focus. 64. Holotype - top grain (34 X 27; 32 X 27 microns), rare diad showing portions of both sides in focus; note coarser reticulum on proximal side, with distal side nearly psilate; SGM-G2. 65. SEM, proximal view. 66. SEM enlargement showing short columellae under reticulum. SEM micrographs by J. W. WALKER.
Plate 6
Figs. 77-84. Placopollis
koobii n. sp.
Figs. 77-84. Placopollis koobii n. sp. . 77. Holotype (66 microns diam.), dispersed tetrad with typical dense wall structure; MGM-X5. 78. Dispersed tetrad (57 microns diam.) with columelloid wall structure visible. 79-80. Same specimen. 79. SEM enlargement showing scabr:ate sculpture and tectal perforations. 80. SEM (66 microns diam.), all apertures trichotomosulcate. 81. SEM (63 microns diam.), all apertures trichotomosulcate. 82. SEM (43 microns diam.), one aperture monosulcate and the other three trichotomo- sulcate. 83. SEM (66 microns diam.), one aperture monosulcate and the other three trichotomosulcate. 84. SEM (71 microns diam.), all apertures monosulcate. SEM-micrographs by J. W. WALKER and R. R. KEITH.
Figs. 85-88. Zonacrinopollis
anasulcatus n. sp.
85-87. Holotype (41X33 microns), proximal-distal compression; SGM-K1. 85. Distal side in focus; note median sulcus and finely reticulate sculpture. 86. Intermediate focus on zonasulclis: 87. Proximal side in focus; note well-developed reticulum supported by columellae. 88. (41 X 30 microns) Specimen broken apart along zonasulcus; note median sulcus on lower (distal) half with finely reticulate sculpture.
Plate 7
Figs. 89-93. Polycolpopollis
magnificus n. sp. ,
89-91. Same grain (76X72 microns). 89. Low focus on two colpi joined to form a loop. 90. Median focus of nexinal body. 91. High focus on third colpus oriented at right angles to the others. 92. Holotype (81X 74 microns) High focus on two slub-parallel colpi that.approach one another at their ends; SGM-S1. 93. SEM (79X 73 microns) Portions of three colpi visible; large reticulum supported by columellae. SEM micrograph by J. W. WALKER.
Fig. 94.
Unidentified fragment. (72 X 60 microns) A portion of a large, possibly
polycolpate species,
perhaps related to Polycolpopollis. Note short broad columellae beneath
a thick reticulum.
Plate 8
Fig. 95. Operculate
clavate monosulcate. (55X30 microns) Icotea-1 well, 3,005-3,024 ft./916-922 m.,
17 miles southeast of Maracaibo, Venezuala, Eocene; possibly arecaceous,
probably
monocotyledonous.
Fig. 96. Bomarea
lycina, extant. (Amaryllidaceae).
(114X66 microns) Note apical auriculae.
Figs. 97-100. Pentecrinopollis
gemmatus n. sp.
97. (38 X 25 microns) Two apical auriculae. 98. SEM (45 X 36 microns), proximal view of in-folded proximal patch - center; one apical auricula. 99. SEM (36X 28 microns); distal view; note resemblance to Reyrea (Early Cretaceous). 100. SEM (39X 23 microns), proximal view of in-folded proximal patch - center; one apical auricula. SEM micrographs by T. D. SHELL.
Figs. 101-104. Steevesipollenites
hemiplicatus n. sp.
101. Holotype (65 X 34 microns), possesses seven non-verrucate ridges, and four verrucate ridges on one side; SGM-V1. 102. (71X27 microns) Possesses seventeen normal ridges, two short ridges, and three verrucate ridges. 103, (52X24 microns) Possesses at least nine non-verrucate ridges on one side and a verrucate patch on the other (proximal?) side. 104. (54X 33 microns) Possesses eleven non-verrucate ridges, four verrucate ridges, and a verrucate patch on one side (proximal?).
Figs, 105-107. Monocrinopollis
doylei n. sp.
105. TEM showing coarsely reticulate-columellate proximal side (on bottom) and foveolate operculate distal side (on top); S = ectosulcus; X 3,290. 106. TEM enlargement of same specimen showing vacuolated non-laminated (dark) endexine folded inside (light) sexine; proximal reticulum on top and distal foveolate sexine on bottom; note loss of footlayer on distal side; X 9,470. 107. TEM enlargemerit of same specimen showing thickened vacuolated endexine underneath aperture, and its thinning laterally to the right of aperture (endexine appears to thin considerably on proximal side under well-developed footlayer); X 9,470. TEM micrographs by J. W. WALKER.
Figs. 108-109. Tricrinopollis
olsenii n. sp.
108. TEM showing coarsely reticulate-columellate proximal side (on bottom and upper right) and foveolate distal side (upper left; note darker homogenous endexine; S = ectosulcus; X 4,690. 109. TEM enlargement of a different specimen showing thicker vacuolated apertural endexine (on bottom) and non-apertural endexine partly detached from footlayer to reticulate-columellate sexine (on top); X22,100. TEM micrographs by J. W. Walker and A. V. Chadwick.
Plate 9
Figs. 110-111. Placopollis koobii n. sp.
110. TEM of tetrad showing transverse sections of at least two grains with massive outer walls and collapsed lumina; X 3,877. 11. TEM enlargement of outer wall composed of irregularly fused rods and granules, and collapsed laminated endexines (white arrows); X 28,788. TEM micrographs by A. V. Chadwick. [Parallel banding is an artifact of thin-sectioning.]
Fig. 112. Tricrinopollis olsenii n.
sp.
TEM enlargement of exine showing dark non-laminated endexine, and footlayer supporting columellae; S = equatorial sulcus, R = reticulum, C = columella, F = footlayer, E = endexine; X 15,184. TEM micrograph by A. V. Chadwick.
Fig. 113. Monocrinopollis doylei n.
sp.
TEM enlargement of exine showing dark non-laminated endexine with many small holes filled with black-stained material, creating a vacuolated texture, and a footlayer on the proximal (lower) side that seems to disappear on the distal (upper) side; note that a continuous (foveolate) tectum forms over crowded and fused granules on distal side; T = tectum, R = reticulum, C = columella, G = granule, F = footlayer, E = endexine; X 27,470. TEM micrograph by J. W. Walker.
AD: Download high quality OG0-092 braindumps to prepare
and pass C4090-451
with green
belt lean six sigma certification. Also get free demos of 642-437 for review of ACCA training.
[12.24.14-17]