Evidence for Plasma Light Sources
by Bruce Cornet, Ph.D.
EMBLA 2001 : The Optical Mission
|
Table of Contents
Ionization and Quantum Light Processes
Plasmas are hot ***ionized*** gases, the atoms of which are having their electrons moved from one atomic shell to another - and that produces light. Their locale on Earth is the atmosphere, which is comprised dominantly of Nitrogen and Oxygen. They are extremely unstable entities, which seek to reach stable states of existence by forming spherical volumes, unless outside forces induce them to behave differently. Without a controlled magnetic environment which allows them to exist in our domain, they will interact with their surroundings to form more stable energy states - which means that they will conform to entropy and disappear! Their existence is dependent on an influx of high energy electrons and quantum particles. For a brief moment in time (depending on the controlling factors) they exist as singularities in an evironment of multiplicities. Plasmas are unique in their characteristics, and do not require identification through any one limited mode of detection. Although they may resemble more conventional sources of energy, such as lamps (energized Tungsten in a near perfect vacuum), they are as different from lamps as elephants are from shrews, or as quarks are from photons.
The following section is from: Unconventional Flying Objects (1995), a scientific analysis, by Paul R. Hill, aerospace engineer, employee of Langley Research Center (LRC), Hampton, Virginia, under the National Advisory Comittee for Aeronautics (NACA) and the National Aeronautics and Space Administration (NASA), July 1939 to July 1970.
Ionization and Quantum Light Processes
"At low altitudes, atmospheric gas molecules such as nitrogen and oxygen consist of two atoms each, like dumbbells, held together by a sharing of their outer electrons. The electrons of such molecules, unless disturbed by a collision with an energetic particle or photon, remain in their lowest energy state, called the ground state. Above the various electron ground-state energy levels are numerous energy-level vacancies. When a sufficiently energetic wave (photon) or particle generated by the UFO collides with a molecular electron in the surrounding atmosphere, the electron is impelled past all energy-level vacancies and outside the molecule. The electron becomes a free entity, rattling around between molecules. The molecule that lost the electron is said to be ionized; it is a positive ion. If the freed electron attaches to a neutral molecule, a negative ion is formed. If a free electron enters a positive ion, it usually enters one of the normally vacant energy levels and gives off a light quanta (photon) having an energy equal to that given up by the electron. Thus a relatively fast electron would give off a relatively energetic photon, say in the ultraviolet, or blue range.
"This electron, occupying what is normally an energy-level vacancy, is in an unstable state. It can't remain because it is attracted toward lower states by the central positive charges. The molecule containing the unstable electron is said to be excited. The electron may cascade down through successively lower energy levels until it arrives at the unfilled ground state, successively giving off light quanta with energies just equal to each change of energy level by the electron. These emissions from the excited molecule depend strongly on the atomic structure and energy-level vacancies of the particular element involved, but are modified by molecular spin. In excited atoms, the energy transitions are distinct, as is the atomic spectral lines. In excited molecules, on the contrary, the temperature-dependent energy of the rotating "dumbbells" is enough to make the spectra appear to be a continuum, having peaks at high energy concentrations and valleys in the frequency regions in between, where fewer Photons are emitted.
"Finally, the energy the electron imparts to each photon determines its wavelength and color. Air molecules can radiate in a kaleidoscope of colors, any color of the spectrum.
"The following equation, in slightly different form, was first used by Einstein in 1905 to explain the photoelectric effect. It is basic to all light phenomena.
"Let E = energy of light photon in electron volts, eV. One eV is the energy delivered to an electron which moves through a 1-volt drop in electric potential.
"l = wavelength of light photon in Angstroms, Å. One Angstrom = 10 - 10 meters.
"Then
l equals 12,400 divided by E, or
l = |
12,400 |
E |
"This equation gives us the following color chart connecting photon energy, wavelength, and color. > means greater than; < means less than.
Photon Energy |
Corresponding |
> 3.26 3.26 - 2.58 2.58 - 2.21 2.21 -2.10 2.07 - 2.00 1.97 - 1.65 < 1.65 |
Resulting l, Å < 3800 3800 - 4800 4800 - 5600 5600 -5900 6000 - 6200 6300 - 7500 >7500 |
Color ultraviolet blue green yellow orange red infrared |
"1. All UFO colors stem from energetic, ionizing radiation or radiations, generated by the UFO, which ionize the air.
"2. Of all the visible colors, red and orange correspond to the least energy, according to this chart. They are also the two most common colors associated with UFO low-power operation, such as hovering or low-power maneuvers. The electrons have been given the ionization energy, but not much more, and cascade down in small energy drops corresponding to red or orange. This is statistically probable, as there are more small drops available than big ones.
"3. According to the color chart, blue requires a relatively high activation energy. Blue, white, and blue-white are the common colors at high-power operation. The blue of the high-power maneuver or high-speed operation corresponds to the strong radiation peaks of nitrogen which will be discussed next. A blend of all the colors tends to white; but with the blues predominating, the blend gives a blue-white, as in an electric are."
Web resources for information on Plasmas.
![]()
http://www.plasmas.com/resources.htm
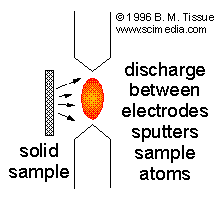
A direct-current plasma (DCP) is created by an electrical discharge between two electrodes. A plasma support gas is necessary, and Ar is common. Samples can be deposited on one of the electrodes, or if conducting can make up one electrode. Insulating solid samples are placed near the discharge so that ionized gas atoms sputter the sample into the gas phase where the analyte atoms are excited. This sputtering process is often referred to as glow-discharge excitation.
http://www.scimedia.com/chem-ed/spec/atomic/emission/dcp.htm
Contained Plasmas (lamps) vs. Free Plasmas
Conventional Plasma Lighting
The most prevalent man-made plasmas on our planet are the plasmas in lamps. There are primarily two types of plasma-based light sources, fluorescent lamps and high-intensity arc lamps. Fluorescent lamps find widespread use in homes, industry and commercial settings.
|
|
High-intensity sources are widely used in industrial and commercial settings as well as for outdoor and security lighting near homes and public areas. It is high-intensity arc lamps that give you the spectacular panoramic views of cities as you fly over them at night.
In high-intensity arc lamps the light we see is generally produced directly by the plasma. Color characteristics are controlled by the chemical elements put into the plasma rather.
Inside every fluorescent lamp there lurks a plasma. It is the plasma that converts electrical power to a form that causes the lamp's phosphor coating to produce the light we see. The phosphor is the white coating on the lamp wall. A fluorescent lamp is shown here with part of the phosphor coating removed to reveal the blue plasma glow inside.
Plasma-based light sources are in fact observable from outer space. Indeed, it may be characteristics of the light from those lamps that tell an alien civilization of our presence.
http://www.plasmas.com/rot-home.htm
Free Plasmas (uncontained)
The plasmas recorded by Cornet were associated with AOP, or aerial objects which moved like aircraft or spacecraft. When recorded using sensitive IR (Infra Red) film, these sources of light appear extremely hot, and unlike lamps of conventional aircraft. On the night Cornet used HSIR film, AOP activity was unusually frequent near Pine Bush, NY.
Picture taken over the Alnoff farm on Flury Rd., Montgomery, NY, on 5 October 1993. Time: 8:39:10 - 8:40:18 pm (68 second time exposure). Film: Kodak High Speed IR (HE) B&W.
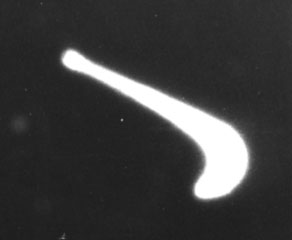
A similar AOP photographed on the same night at 8:58 pm clearly shows a hot gas aura (fuzzy margin) around an even hotter plasma light. As the light dimmed to the right, it did not go out, but instead grew more faint and intermittent - unlike the behavior of conventional lamps. If a lamp on a conventional aircraft becomes blocked by some part of the fuselage as its orientation changes, the light will seem to go out. It will not produce an intermittent trail of heat as in the image below.
Kodak High Speed IR film (enlargement of negative):

Below: Another similar performance photographed with 400 ISO Konica color film. Picture taken at bend in West Searsville Rd. on 28 April 1993, 9:03 pm. Note the glowing blue (orange in positive print) aura around central white light when it became brighter, and the round bluish-white and reddish-orange strobes.
Enlargement of negative (positive insert).
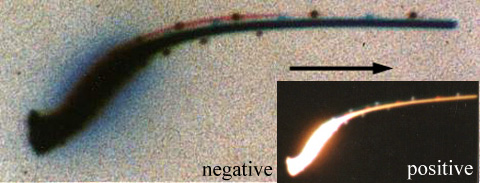
For the skeptics: If you think this is a photograph of a conventional aircraft changing orientation to the camera, and the blue aura is only glare caused by its landing light pointing at the camera lens, look at the picture that was taken immediately before this one at 9:01 pm. Please do explain how the vortex-like feature was produced using conventional technology, which shows the strobes displaced around a spiraling light.

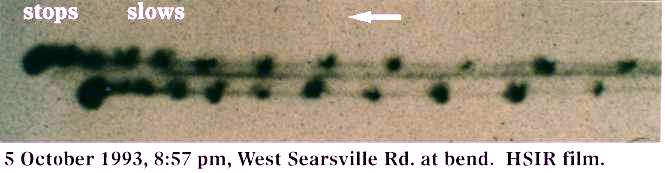
On that same night (5 October 1993) Cornet photographed an AOP as it slowed to a stop in midair. It had a small white belly light, two small white outboard lights, and a strobe embedded in each outboard light. The strobes fired with an unusual irregularity in intensity (unlike conventional aircraft strobes) and produced irregularly-shaped heat splotches on the IR film instead of round dots (as a conventional aircraft strobe would produce - the light shape being controlled by the lamp shape). The strobes of an AOP can appear as discrete dots, which may indicate either different types of plasma technology or human technology at work. The possibility of hybrid human technology, which contains reverse-engineered foreign technology is not out of consideration.
As the AOP slowed down (see image below), its strobes become more closely spaced, until they become superimposed after it stopped. That is why the last strobes on the left of this image are larger: The irregularity of the strobe shape averaged out to produce two larger and brighter spots on the film. This AOP waited until Cornet closed the shutter of his camera, and in the short interval of time it took for the film to be advanced by a motor drive and for Cornet to open the electronic shutter again (about 1 second), this AOP dove into the ground and disappeared!
Kodak High Speed IR film:
At 10:10 on the same night, Cornet photographed the following light doing a complex spiral. Note the intricate corkscrew pattern, which cannot be duplicated by camera vibration or movement. This pattern is consistent with the patterns of spirals and loops for other performances, such as in Special Agent 707 and in The Transformation.
Kodak High Speed IR film:
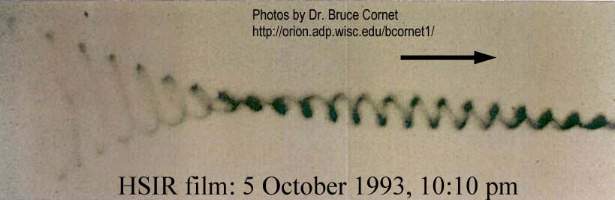
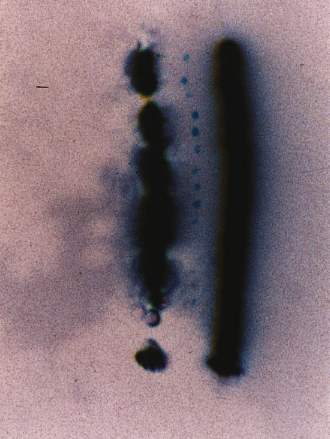
But the most convincing evidence for plasma lights comes from plasma orbs.
On 4 September 1992 Bruce Cornet, Ellen Crystall, and Fred Brock were at the Jewish Cemetery on Rte 52 between Walden and Pine Bush, NY (see "Oh sure!" web page on this sighting). That area was a favorite place to watch for AOP at night (Crystall, 1991, p. 72). At 11:20 pm a set of lights descended from the northwest to a corn field on the north side of Rte 52 opposite the cemetery, and proceeded to move west low to the ground (see map). At the western side of the field, just before the field ended in a forest, the lights turned and began coming at the observers. Crystall captured the event on video, while Cornet took time exposures. As the AOP approached, two bright lights could be seen, with a flashing red light in between them. The AOP began to climb and flew over the observers. Its shape was that of a diamond (manta shape).
As the AOP first began its approach to the observers, its right light flickered. The first time exposure shows something rather extraordinary. While the left light appears to be continuous and unbroken, the right light sputters and momentarily goes out a few times, creating a discontinuous light trace on the photograph. One can clearly see what appear to be fumes coming off of both lights, but because the right light is discontinuous, the fumes are also discontinuous. If that light had been continuous like the left light, it would be difficult to distinguish the difference between fumes or substance coming off the light versus simple glare. Each time exposure is numbered in sequence, and the artificial break between them shown.
Note the orbs or spheres present near the bottom of the right light trace. When the negative of this image was photographed under a microscope and enlarged (below), the gases produced around the lights became very evident.
Enlargement of plasma orbs below.
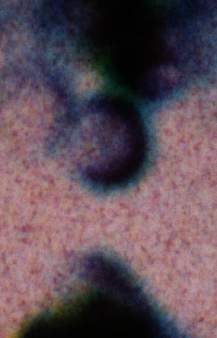
Without spectral data on these types of lights, which would provide unequivocal proof of their composition, their interpretation as plasma gases must be based on other information. In 1994 this image was shown to physicist Dr. W.C. Levengood, whose laboratory is located near Detroit, Michigan, U.S.A. Among other things, Dr. Levengood is a plasma physicist. He recognized the orbs as balls of highly charged plasma based on their appearance, and association with the orange-brown Nitrous Oxide gases.
When the atmosphere is ionized, the two most common elements, O2 and N2, combine to form various oxides of Nitrogen (laughing gas). Oxides of Nitrogen are produced naturally by lightening. Commercially, NO2 is produced by an electrical arc (controlled lightning). Oxides of Nitrogen are produced only by one form of life on Earth: Nitrogen-fixing bacteria (Rhizobium). When dilute, the gas is almost colorless, but when concentrated it becomes brown. Its color is directly proportionate to its concentration. For the air around the AOP lights to be as orange-brown as in the pictures, the concentration of NO2 must have been very high. Whether or not the discontinuity of the right light was intentional, the result was the creation of evidence which not only proves that these lights are not conventional lamps, but also demonstrates their difference from chemical flares (e.g. fireworks below).
Chemical flares can be designed to give off various colors, depending on the elements added to the explosive mix (usually metals with available electron shells). Typically, the red color of road flares and fireworks is more pinkish red than red. In the images of fireworks below, when the initial charge detonates (ovoid blobs in lower left corner) nitrates in the explosive are instantly decomposed. When they decompose they produce NO2 gases from -NO3 breakdown. It is that color (below) which most compares with the aura around the AOP lights, now isn't it? But there is a major difference between these two similar occurrences of concentrated NO2: In one case there was an accompanying loud explosion ; in the other case there was silence. What sound do you think came from the AOP?
Other differences between chemical flares and plasmas: There is a broad area (or many microsites) of combustion (even for road flares) rather than a central area (source) of heat and light as in plasmas. Because the atoms of plasma gases are highly charged or polar, they attract and repel one another, forming spherical balls or orbs. The smoke of flares and fireworks tends to reflect and obscure light, rather than glow as in the AOP example. But most importantly, there is no evidence in chemical sources of light of spherical cohesion and the formation of perfectly round glowing orbs. If one studies the interrupted light source of the Manta Ray (above), you can actually see that the plasma stream breaks up into a crowded mass of light brown plasma orbs in image #2 (right side of photo), but when the plasma stream becomes brighter (wider), the orbs join to form a continuous plasma field.
Nevertheless, additional data from independent methods are required to eliminate all alternate explanations.
Copyright B. Cornet 1999
Crystall, Ellen, 1991. Silent Invasion: The shocking discoveries of a UFO researcher, Paragon House, NY.
Hill, Paul R., 1995. Unconventional flying objects, a scientific analysis, Hampton Roads Publishing Co., Inc., Charlotesville, VA.
Date this web page was last modified: 12/02/07