Return to First
Half
Last
updated on 05/05/2007
More
Publications (1973-2006)
When Did Angiosperms First Evolve?
continued
by Bruce Cornet, Ph.D.
Table of
Contents
Abstract
Introduction
A Mystery
Compounded by Assumptions
Angiosperm
Pollen Morphology and Wall Structure
The Oldest
Cretaceous Angiosperm Record
The
Thigmomorphogenic Hypothesis by John Miller
New Challenges and Opportunities for Innovative Taxonomy
by John Barrett
More Questions about a Cretaceous Origin
The Angiosperm Invasion of Gondwana
Paleoequatorial Climate During the Jurassic
Super Greenhouse in Early Jurassic
Basal Angiosperm Hierarchy
Molecular Evidence and Fossil Evidence Combined
Water Lilies? in the Basal Jurassic
The Triassic
Angiosperm Pollen Record
Pseudo-Tricolpate Pollen
Beetles - Incidental Pollination
Cheirolepid Conifer Adaptation
Competition with Cheirolepidaceous Conifers
Second half
Radical Changes in Pollen Morphology and Exine Structure -
implications
How Rapid Evolution Might Occur - a molecular perspective
The Threshold of Angiospermy
What Factors Caused the Delay in Angiosperm Radiation?
Evolutionary
Reversals and Archived Genes
The Significance of
Polyplicate Pollen
The Problem:
Recognition of Pre-Cretaceous Angiosperms
Implications
of Pre-Cretaceous Fossils
Key angiosperm synapomorphies
Sanmiguelia lewisii
Angiospermous
characteristics
Multilacunar
node and growth habit
More Evidence for Late Triassic Angiosperms
A Mid-Jurassic Angiosperm? with
Monocot-like Leaves
Schmeissneria from early Late
Triassic? to Middle Jurassic
A Living 'Dicot' with Monocot-like
Leaves!
Origin of
Bisexual Flowers
Basal Angiosperm Root Parasites?
Endosperm Development
Dicot-like Leaf Venation
Why The Conflict Between
Geneticists and Paleobotanists?
Popular Theories Die Hard
Conclusions
References
Glossary
Addendum: What About Lequeria elocata?
Radical changes in pollen morphology and exine structure
They signal changes in reproductive biology.
There are two types of incompatibility systems known in seed plants, which reduce the amount of self pollination for monoecious plants (ones that produce both male and female gametes - a condition found in all bisexual flowers). The most primitive method is gametophyte (haploid) incompatibility, which occurs during meiosis in gymnosperms. Sporophyte incompatibility systems evolved in plants which enclose their ovules/seeds in an ovary, thereby placing another layer of protection between gametes. Because this new layer of incompatibility protection is part of the diploid sporophyte (the plant we see), the method is called sporophyte incompatibility. Substances involved in effecting incompatibility (preventing a plant from pollinating itself) are typically added to the outer part and/or spaces inside the wall of pollen grains. Angiosperms utilize the spaces created by columellae in the exine for incompatibility recognition chemicals, and the holes in the tectum (outermost wall layer) provide a path for these chemicals to interact with receptors on carpel stigmas. When those holes are lost during the evolution of a tectate-imperforate exine in angiosperms, the sporophytic incompatibility system becomes inoperable or ineffective. Self-compatibility results. It characterizes groups within the Magnoliidae with highly elaborate beetle-pollinated flowers that have evolved intricate mechanisms to minimize self-pollination (Endress, 1990).
The appearance of a sporophytic recognition or incompatibility system might be implied by exine structure in the Crinopolles Group, if structure and function are related as they are in angiosperms. Such a system could enhance genetic outcrossing and distribute non-lethal mutations throughout an interconnected gene pool, thereby increasing diversity at the subspecies level. But wide distribution also has the effect of stabilizing a population by diluting the effects of any one gene/character change. Isolation and speciation are thought to occur only in fringe populations cut off from the main gene pool, and it is mainly in those smaller populations that the effects of mutations could be adequately tested with species survival or extinction.
How Rapid Evolution Might Occur
Changes resulting from the rearrangement and/or inversion of segments on chromosomes could cause larger genetic changes than point mutations, and will not activate the process MSUD "meiotic silencing by unpaired DNA." Recently, Shiu et al. (2002) showed that inclusion of unwanted viral genes and insertion sequences will stop the meiotic process if there is unbalanced gene pairing.
""In meiosis, normal chromosomes pair with one another perfectly," noted Stanford Research Professor Robert L. Metzenberg, co-author of the Cell study. "We discovered that, when chromosomes pair, there's a built-in checking system we didn't expect to find that checks if the pairing is correct. It does not detect tiny differences in the two DNA sequences, but any deviation the size of a gene or larger triggers the checking system."
"Three copies distributed between two parents is sure to make trouble, Metzenberg said, because one copy is likely to be unpaired, but four genes are not necessarily bad because they can pair normally and do not trigger the MSUD checking mechanism.
""If there's a gene missing or appears in one chromosome but not in its mating partner, the cell says, 'Something is wrong. There's something from one of the parents that doesn't belong there,' " he added." (Shwartz, 2002).
The silencing of the MSUD, however, allows for interspecific crosses, which could explain how major changes in the genetic code could occur in just a few generations.
"Likewise several species of Neurospora N. crassa, N. sitophila and N. tetrasperma are normally infertile when crossed in the laboratory. Yet, by including a dominant mutant gene called Sad-1 in the DNA of the three mold species, Shiu and his colleagues were able to produce viable spores through cross-breeding of species that normally are sterile with one another.
"The ability of Sad-1 to breach interspecies sexual barriers apparently works by preventing meiotic silencing from occurring, according to Metzenberg.
""To our knowledge, this is the first case where the barrier between interspecies crosses has been observed to break down as a result of mutation in a single gene," Shiu added, "but since gene silencing is universal, it could occur in other kingdoms, including plants and animals."" (Shwartz, 2002; Shiu et al., 2002)
Davidson (2000) presents additional insight into speciation in his new hypothesis for rapid organic change (the semi-meiotic process).
"Consider an oöcyte about to undergo meiosis. Assume further that this oöcyte has one chromosome which has undergone an inversion. When the tetrad is formed at synapsis (Figure 1. A diagram to illustrate the instantaneous formation of a new chromosome pair following the occurrence of a single pericentric inversion. O represents the centromere.) it will consist of two daughter chromosomes (sister strands or dyads) of original structure and two daughter chromosomes each containing the inversion. In the first meiotic division one pair of sister strands is discarded into the first polar body leaving the other pair in the egg. If the inverted pair is discarded, the oöcyte retains the original genome. If the normal pair is discarded the oöcyte has instantly acquired a new karyotype in homozygous form and, following the duplication of the centromere (which results in the separation of the chromosomes) an evolutionary potential as a new kind of diploid organism.
"All genetic (evolutionary) changes originate in individual cells, in individual chromosomes, in a particular organism. The evolutionary significance of the individual will become apparent in a later section. If the inversion in the above example occurred in a cell destined to become part of the female reproductive lineage, then at the termination of the first meiotic division, one half of the products of that lineage will be like the original and one half will be a potentially new kind of organism with a new paired (homozygous) chromosomal genotype (karyotype) produced in a single cytogenetical step. In this system the only requirement is for one or more oögonia to be heterozygous. Such an evolving series would be expected to produce a number of discrete products determined by the number of chromosome rearrangements involved in the series. I do not suggest that all new chromosome homozygotes would be new species. In fact we know that is not necessarily so. Nevertheless, this perspective is worthy of further attention."
The Threshold of Angiospermy
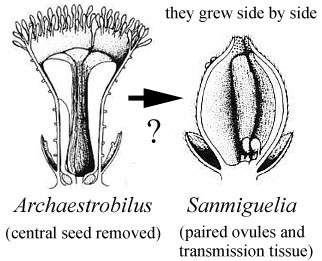
Could the changes we see taking place in Steevesipollenites and Crinopolles pollen have been premature or ineffective in the Carnian (Late Triassic)? Or perhaps the changes in exine structure were triggered by carpel closure, requiring a recognition mechanism to evolve so that a pollen grain would "know" when it should germinate. Later that mechanism could evolve an incompatibility aspect to reduce selfing. Cornet (1989a; 1996) showed that a coeval plant (Pelourdea - Archaestrobilus), living in the same semiaquatic environment as Sanmiguelia, might contain clues as to how the ascidiate (barrel-shaped) carpel and stigmatic area evolved in a step-wise gradual fashion under selective pressures from insects (especially beetles). See John Miller's Thigmomorphogenic Hypothesis.
Above: Illustrations (reconstructions) of Archaestrobilus cupulanthus and Pelourdea poleoensis from Cornet, 1996.

Above: Leaves of Pelourdea poleoensis attached to a stem (discovered in Big Indian Wash, UT, by Paul Olsen; photo by Paul Olsen, 1984).
Above (modified from Ash, 1987; Tidwell et al., 1977): a. Reconstruction of Pelourdea poleoensis; b. Reconstruction of Sanmiguelia lewisii.
The ascidiate (barrel-shaped) carpel is now considered basic to angiosperm carpel evolution (Taylor and Kirchner, 1996). Formerly the conduplicate carpel of the Magnoliales was considered basic (Fig. 6.5n). The carpel type in Sanmiguelia (Axelrodia) is that illustrated in their Fig. 6.5o below (colored green), which is considered basal but derived by Taylor and Kirchner (see figures showing Sanmiguelia carpel).
Ovule Evolution. It is striking that outgroups have orthotropous ovules; yet, in angiosperms, orthotropous ovules are relatively rare and only weakly correlated with ascidiate carpels and ovule numbers of one or two (Taylor, 1991a). In addition, orthotropous ovules are variably placed in relation to the gynoecial appendage. If we start with the proposed ancestral carpel (Fig. 6.5A, C) and the ancestral orthotropous ovule (Taylor, 1991a) we can propose three directions in which these organs could have adapted to place the micropyle further from the stigma and transmission tissue. One strategy would be a curved ovule with the micropyle directed basally (Fig. 6.51). A second would be to move the orthotropous ovule to a more lateral or apical position (Fig. 6.5E). A last strategy would be a terminal stigma (Fig. 6.5B). Thus, selection for increased micropyle-stigma distance would result in new carpel forms as well as other ovule types. (Taylor and Kirchner, 1996: 135)
Figure 6.5 from Taylor and Kirchner (1996): "Diagrammatic representations of carpel types containing the two major types of ovules, showing their evolutionary relationships. The proposed ancestral types, ascidiate with basal to slightly lateral placentation, are on top. Note that the carpels with anatropous ovules, or with ascoplicate or plicate morphology, are inferred to be derived. Dark arrows show initial evolution of morphologies having orthotropous ovules. Open arrows show evolution of derived carpels with anatropous ovules."
The stigmatic (pollen-receptive) surface and pollen-tube transmission tissue evolved from sterile scales inside a cupulate organ, which was also ascidiate in shape (Cornet, 1996). Evidence for this comes from Anthurium (Araceae), which may still possess remnants of those sterile scales. They are fused to form a branched organ with tufts or brush-like heads at their distal ends. This organ is centrally located, free standing, and has bitegmic ovules attached to its base (three to four ovules in a common ovary). The gynoecium is syncarpellate, with communication between the three carpels. There are only two stalks with brush-like heads rather than three, implying an independent origin rather than a derivation from the fusion of three carpels. The ovary is filled with a gelatinous substance (similar to that of Amborella), and the apical part of the pistil is open so that the brush-like heads of the transmission tissue can be exposed for direct pollen reception. In other words, the gynoecium of Anthurium is not closed, and may still possess evidence for the evolution of transmission tissue from sterile homologues of ovules. Molecular data now place the Araceae near the base of the Monocots.
Above: Placenta, ovules, and transmission tissue were dissected out of an Anthurium gynoecium as a separate and free-standing organ system, attached to the fused carpels only at their common base.
Cupulate reproductive organs are known in Bennettitalean flowers, once considered a sister group to the Anthophytes, or angiosperm ancestors related to the Gnetales. With more discoveries, cupulate organs will probably emerge as a fundamental aspect of stem angiophytes. Archaestrobilus may be related to angiosperm ancestors and could be a Permian survivor. Archaestrobilus cupulanthus bears an uncanny resemblance to the Magnolia strobilus (fruiting stage), once considered evidence for a gymnospermous ancestry of angiosperms (see photo and reconstruction above).
The evolution of a sporophytic incompatibility system is important, but it's suppression is equally important. Suppression through exine reduction would have the effect of increasing self-pollination, isolating variation, and enhancing genetic stability (almost all gymnosperms - with the possible exception of some Cheirolepids and Gnetales - lack a sporophytic incompatibility system, which correlates with their conservatism).
The loss of a self-incompatibility system in extant angiosperms is considered a derived characteristic. Alternative isolation mechanisms to self-incompatibility have evolved in the Magnoliidae, and are quite important, such as dichogamy and a frequent shift to monoecy and dioecy. The regular occurrence of unisexual flowers is known from at least 21 families of Magnoliidae (Endress, 1999: 16).
What Factors Caused the Delay in Angiosperm Radiation?
How important was the mass extinction event at the Triassic-Jurassic boundary in delaying an angiosperm radiation? Tanner et al. (2003) show that disruptions in plant communities were not focussed at the boundary, but occurred through the Norian and into the Early Jurassic (Hettangian), with only three plant families completely disappearing: Glossopteridaceae, Peltaspermaceae, and Corystospermaceae (Ash, 1986; Traverse, 1988). Was there something about Jurassic climates that promoted monotypic floras with very large geographic distributions? Was plant innovation retarded or redirected in the Jurassic by climatic pressures (mutations in angiosperms were restricted in the Jurassic to 18S rather than to rbcL positions: Sanderson and Doyle, 2001), and did it take much of the Jurassic for floral diversity to completely recover? These are questions paleobotanists would not have asked prior to 1994 when one of the first papers on bolide-induced mass extinction at the Triassic-Jurassic boundary was published (Fowel et al., 1994). Could factors such as mass extinction, climatic deterioration (e.g. the desertification of paleoequatorial regions during the Early Jurassic and loss of extensive rain forests), and new insect vectors played a role in the timing of a sustained angiosperm radiation in the Cretaceous (coordinated across familial lines)? These are the only reasonable alternative explanations to a Cretaceous origin (Occam's Razor applies).
Below: Postulated history of angiosperm evolution in the Mesozoic, showing the initiation of origin (Triassic) and the initiation of radiation (Cretaceous) related to the sequential breakup of Pangaea and the coincidence of rift basins (extremes in topography and isolation) in a wet tropical climate.
Click on figure below for enlargement.
Data in above graphic modified from Hickey & Doyle (1977); Doyle et al. (1977); Cornet and Olsen (1993); John C. Butler (1995); Alister Rees (2001); Hochuli and Feist-Burkhardt (2004).
Evolutionary Reversals and Archived Genes
Lily-like pollen is surprisingly well represented in Early Cretaceous palynofloras (Doyle, 1973), and has been traced through Stellatopollis back to the Oxfordian (e.g. Cornet and Habib, 1992). Even the monocot pollen of Calectasia cyanea (Xanthorrhoeaceae: Asparagales) of south and west Australia preserves the compound monosulcate condition found in the Crinopolles Group through its undeniable tetrasulcate condition (Chanda et al., 1978).
Auriculae are bulbous exinal protuberances found on certain Triassic, Cretaceous, Tertiary, and Recent pollen. Normally auriculae occur at the apical ends of pollen grains, but can be widely distributed as sculptural elements (clavae) over the entire pollen grain. They tend to occur mainly on Triassic to Cretaceous (Mesozoic) polyplicate pollen types, but also on Tertiary to Recent monocot pollen types. Auriculae may be synapomorphic for angiophytes (i.e., stem-group + crown-group angiosperms), representing expressed or non-expressed (archived) genes that survived in some crown-group and Recent angiosperms.
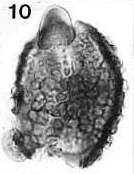
 Ricciisporites umbonatus Felix and Burbridge (1977) is a Late
Triassic index fossil for the Carnian and Norian of the Canadian Arctic, while R.
tuburculatus Lundblad (1954) is an index fossil for the Rhaetian. This
palynomorph type may be a member of the basal Crinopolles group of early Carnian age
(similar to Pentecrinopollis gemmatus - image 97 below). They can be
compared to similar morphotypes in the Cretaceous and Tertiary - raising the possibility
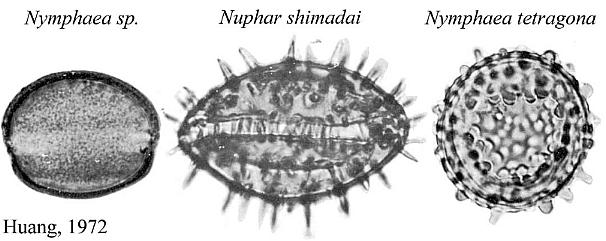
that Nymphaea (see N. tetragona pollen below) might be a living
descendant.
Ricciisporites umbonatus Felix and Burbridge (1977) is a Late
Triassic index fossil for the Carnian and Norian of the Canadian Arctic, while R.
tuburculatus Lundblad (1954) is an index fossil for the Rhaetian. This
palynomorph type may be a member of the basal Crinopolles group of early Carnian age
(similar to Pentecrinopollis gemmatus - image 97 below). They can be
compared to similar morphotypes in the Cretaceous and Tertiary - raising the possibility
that Nymphaea (see N. tetragona pollen below) might be a living
descendant.
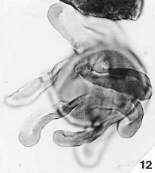
Place pointer over image below for an identity and age. For Mac users, from upper left to lower right: Steevesipollenites hemiplicatus (Carnian), Pentecrinopollis gemmatus (Carnian), Ricciisporites sp. (Albian), Elaterocolpites castelainii (Cenomanian), operculate clavate monosulcate (Eocene), Bomarea lycina (extant).
 |
 |
 |
 |
 |
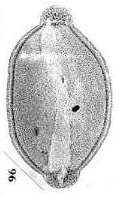 |
Nelumbo nucifera (Sacred lotus) and Nelumbo lutea (American lotus) - family Nelumbonaceae - are members of the order Nymphaeales, the aquatic water lilies. Based on molecular data, Martin et al. (1993, p. 154) consider Nelumbo to have an extremely ancient separation from all other angiosperms, including the Magnoliidae. Doyle (1998) places the Nymphaeales at the base of his cladograms (see Figure 4 below). Nymphaea produces mostly monosulcate and zonasulculate pollen which does not have the distinctive reticulate-columellate wall structure of Nelumbo. The sculpture on Nymphaea pollen ranges from granular to echinate to clavate-gemmate as in Pentecrinopollis gemmatus. The reticulate-columellate condition of Nelumbo pollen is thought to be derived, but if it is basic to angiosperm exine evolution, as Crinopolles pollen suggests, either Nymphaea pollen has lost its tectate wall structure, or it represents a pre-tectate (pre-Crinopolles) state like that of P. gemmatus. The Crinopolles position (cladistic node) would occur just above the branch for the Nymphaeaceae, making Nymphaeales and Amborelaceae pollen pre-reticulate-columellate.
 |
Significantly, Nelumbo pollen varies from trisulcate with a pair of equatorial sulci positioned as in Tricrinopollis (Cornet, 1989a) to tricolpate (Kuprianova, 1979) - the aperture type which is basic to the Cretaceous angiosperm radiation (Doyle, 1977). Nelumbo keys out on Doyle and Endress's (2000) cladistic-molecular tree above the Chloranthaceae and at the base of the Eudicots.
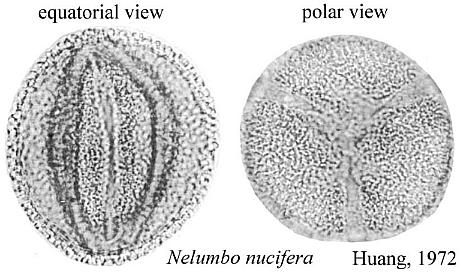 |
The equatorial sulci occasionally join in Nelumbo to form a ring sulcus, as in Zonacrinopollis anasulcatus from the Triassic. Zonasulculate pollen is also common in the Nymphaeaceae (see N. tetragona above).
Kuprianova regards the trisulcate condition in extant Nelumbo pollen as a vestige of the palynological transition from monosulcate to tricolpate dicot pollen. But Crinopolles pollen shows the exact reverse. Crinopolles evolution deduced by Cornet (1989a) from morphologic and stratigraphic analyses reveals an alternative hypothesis: Tricolpate pollen need not be derived from a monosulcate precursor and the monosulcate condition can be derived. Tetrad orientation ranges from radial to bilateral in both Nelumbo and Tricrinopollis, indicating that both morphotypes are ideal precursors to tricolpate pollen. One is Extant. The other is Triassic. The problem Cretaceous paleobotanists seem to have is reconciling these facts with their Cretaceous origin and radiation theory.
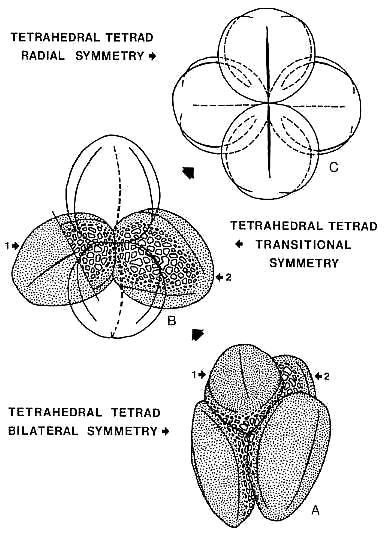
The morphological similarities between Nelumbo pollen and Tricrinopollis spp. raise the distinct possibility that Nelumbo pollen, along with Calectasia pollen, may actually be Crinopolles survivors (i.e., the genes were suppressed and archived, and later reactivated).
The Significance of Polyplicate Pollen
Cornet (1989a) showed that the polyplicate precursors of various types of reticulate-columellate angiosperm-like pollen in the Triassic have analogs, if not homologues, in various families of monocots. The polyplicate, inaperturate, monosulcate, disulcate, and zonasulculate pollen (including the entire range of sculptural types) found in the six tribes of the Araceae (Thanikaimoni, 1969) all have analogs in Triassic, Jurassic, and Cretaceous angiosperm-like pollen, making the morphologic level of pollen evolution in this family the most primitive of any other monocot or dicot family. And yet because the monocots have been considered derived from dicots usually above the level of the most primitive extant magnoliidae (including the Piperales and Nymphaeales), or above Eudicots, aroid pollen anomalies are discounted or considered derived by botanists. In fact, the Aroideae (and Philodendroideae) are placed by some botanists systematically above all other Aranae families (indicating that they are the most derived monocots in that branch). In contrast, molecular results and new cladistic trees show the Liliflorae, Ariflorae, Triuridiflorae, and Alismatiflorae to have diverged first, in that order (Herendeen and Crane, 1995).
Friis et al.
(2004)
recently published (November 2004) a paper on Araceae flowers (Mayoa portugallica)
and pollen from the Early Cretaceous (Barremian-Aptian) of Torres Vedras in the Western
Portuguese basin. The pollen
resembles that of extant Holochlamys more than it does that of Spathiphyllum,
in that its outer exine (sculpture) has two fields of parallel ribs that converge at
opposite ends. 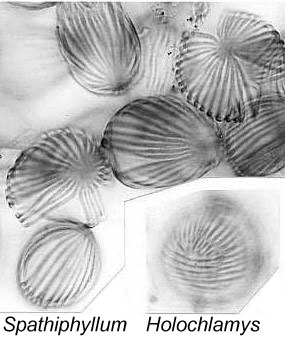 Each field occupies about one half of the pollen grain, and is rotated up to 90
degrees from the field/orientation on the opposite side of the grain - similar to that
of Cornetipollis from the Late Triassic, Multimarginites from the
Oxfordian (Late Jurassic) and Tertiary, and extant Sanchesia (Acanthaceae; see
Vasanthy et al., 2004). Several other plant fossils from the Early Cretaceous of
Portugal are described, which may have alismatalean or even araceous affinity.
Zona-aperturate pollen from a coprolite has a punctate-foveolate tectum and an aperture
that is equally wide for its full length. Such pollen is known only for members of
two araceous subfamilies: Monsterae (e.g. Monstera) and Zamioculcadeae (e.g. Gonatopus).
Similar Monstera-type of pollen has been recovered
from latest Triassic strata in the Newark basin of New Jersey. Although these
authors are reluctant to push monocot divergence much before the Albian (Herendeen and
Crane, 1995), the existence of such "derived" monocots that early in the
Cretaceous radiation supports the idea that the first monocots are substantially older
(i.e., pre-Late Jurassic: Cornet
and Habib, 1992).
Each field occupies about one half of the pollen grain, and is rotated up to 90
degrees from the field/orientation on the opposite side of the grain - similar to that
of Cornetipollis from the Late Triassic, Multimarginites from the
Oxfordian (Late Jurassic) and Tertiary, and extant Sanchesia (Acanthaceae; see
Vasanthy et al., 2004). Several other plant fossils from the Early Cretaceous of
Portugal are described, which may have alismatalean or even araceous affinity.
Zona-aperturate pollen from a coprolite has a punctate-foveolate tectum and an aperture
that is equally wide for its full length. Such pollen is known only for members of
two araceous subfamilies: Monsterae (e.g. Monstera) and Zamioculcadeae (e.g. Gonatopus).
Similar Monstera-type of pollen has been recovered
from latest Triassic strata in the Newark basin of New Jersey. Although these
authors are reluctant to push monocot divergence much before the Albian (Herendeen and
Crane, 1995), the existence of such "derived" monocots that early in the
Cretaceous radiation supports the idea that the first monocots are substantially older
(i.e., pre-Late Jurassic: Cornet
and Habib, 1992).
Above left: Mayoa portugallica, in situ monocot pollen from the Early Cretaceous (Barremian–Aptian) of Torres Vedras in the Western Portuguese Basin (Friis et al., 2004). Above right: Equisetosporites chinleanus from the Carnian-Norian Chinle Formation of Arizona and New Mexico (Cornet, 1993). These types of pollen have been erroneously classified as spores of Equisetum (a fern ally) or as the pollen of the Gnetales. E. chinleanus has been recovered in situ from the enigmatic Dechellya gormanii (Ash, 1972), a possible stem angiophyte.
But could the key characteristics used in taxonomic classification be interpreted differently? In computer words: Wrong assumptions in, wrong conclusions out. For example, Doyle (1998) indicates that the parallel venation of monocot leaves is a derived character (see diagram below), which is necessary if monocots are derived within the crown-group. Sanmiguelia (Late Triassic) with its parallel-veined leaves and multilacunar nodes would challenge that interpretation if it is not a crown-group angiosperm. Not all botanists, however, hold the view that monocots are derived (e.g. Heresy Revised: the monocot theory of angiosperm origin: Burger, 1981). Perhaps the most primitive living monocots and dicots cannot be linked to one another in a simple ancestor-descendant fashion, because the 100-300 million-year-old angiosperm roots, which once joined them (compare Amborella and the Nymphaeaceae), are either missing from the picture or haven't been figured into the puzzle yet (e.g. molecular data and Sanmiguelia).
More recent work by Doyle and Endress (2000) and Doyle (2001) revise taxonomic relationships using new molecular data.
Angiosperm crown-group radiation from angiophyte ancestors.
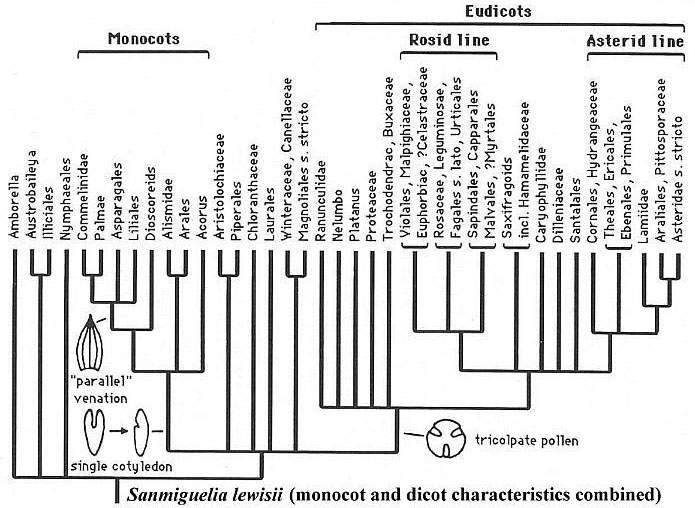
Modified from Doyle (1998, Figure 4).
The Problem: Recognition of Pre-Cretaceous Angiosperms
Recognizing fossils of extinct Jurassic angiosperms may be difficult if preconceptions based on extant angiosperms influence judgement. Just ask the question: What might an angiosperm more primitive than any living group of angiosperms look like? What if it combines characteristics which separate different families and orders of angiosperms today, but does not support any established model for angiosperm evolution? More importantly, what if that Jurassic angiosperm still possessed some characteristics of the ancestors of angiosperms, characteristics which could be used by the skeptic as evidence it is not an angiosperm?
"Archaefructus presents a new set of characters not previously known in angiosperms. Typically, the division Magnoliophyta (25) is used for angiosperms or flowering plants, and the class Magnoliopsida is used for the dicotyledons and Liliopsida for the monocotyledons. We suggest that a new subclass, Archaemagnoliidae, be constructed for angiosperms that do not conform to the character sets of any of the existing subclasses of the Magnoliophyta. This new subclass is characterized by flowers subtended by only a single leaf or leaf-like organ. Flowers consist of elongate receptacles bearing conduplicate carpels helically. The nature of the male floral organs is unknown at this time. Flowers appear to terminate axes and predate the evolution of any floral patterns. The subclass does not fit the concepts of "paleoherb" or of "eoangiosperm," as both represent collections of angiosperm taxa already more specialized and modified (26) than Archaefructus of the subclass Archaemagnoliidae." (Sun et al., 1998).
Is Archaefructus just such an example? Does it represent an extinct angiosperm lineage, one which retains a hint of ancestral characteristics and a link to the Gnetales and/or seed ferns? Or will the age of the "Jianshangou Bed" be shifted according to how Archaefructus is classified (e.g. Sun et al., 2002)? The recent disclosure of paired stamens located below conduplicate carpels on petal-less reproductive axes shows Archaefructus to be much more specialized (derived) than Sanmiguelia, for example (Sun et al., 2002). Resembling the tectate-granular pollen of Sanmiguelia, Archaefructus produces atectate monosulcate pollen, which is assumed by angiosperm paleobotanists to be the basic (oldest) pollen type for angiosperms (see above).
The usual position taken on pre-Cretaceous fossils with angiospermous characteristics is: "There are several recent reports of Triassic, Jurassic, and lowermost Cretaceous-aged fossils identified as angiosperms (1-4), but none of these reports can be accepted as conclusive evidence for the presence of angiosperms." (Sun et al., 1998).

Jim Doyle with Ascarina lucida (Chloranthaceae) in New Zealand, 2003.
Doyle and Donoghue (1993) give one of the best and possibly most balanced evaluation of pre-Cretaceous evidence for its time:
"Implications of Pre-Cretaceous Fossils
"The alternatives we have outlined would be easier to evaluate if we had direct evidence on the status of the angiosperm line before the Cretaceous. Better information on the diversity of other anthophytes might also help; most of our knowledge is based on a few specialized taxa, and it is possible that other less well-known forms have character combinations that would modify ideas on basic states in outgroups and therefore in angiophytes themselves.
"There is no lack of supposed pre-Cretaceous angiosperms (Axelrod 1952; 1970), but most have been shown to be unrelated to angiosperms, incorrectly dated, or possibly related to angiosperms but lacking sufficient diagnostic characters for interpretation (Scott et al. 1960; Hughes 1961; 1976; Hickey and Doyle 1977; Doyle 1978). However, there have been several promising recent discoveries and new insights on older ones. Again, our point is that consideration of how such fossils fit into phylogenetic trees is of prime importance in evaluating their significance. A critical question in each case is whether the fossil has angiosperm states in all its known characters, making it a potential member of the crown-group, or a mixture of angiosperm advances and more plesiomorphic states, placing it along the stem-lineage. Depending on how completely they were known, fossils of the first sort would constitute evidence that node B had been reached (fig. 15a). Fossils of the second sort might mean that the crown-group had not yet originated (fig. 15b), although it could not be excluded that the crown-group existed at the same time but has not been detected (fig. 15c).
"Unfortunately, none of the fossils in question fall unequivocally in either category. However, the best candidates are all suggestive in showing states more like paleoherbs than woody magnoliids. If they are angiosperms, they could mean either that paleoherbs are primitive, or that angiosperms had split into paleoherbs and woody magnoliids but the latter have not yet been found; if they are stem angiophytes, they would definitely favor the view that paleoherb features are primitive in angiosperms." Doyle and Donoghue (1993, p. 161-162).
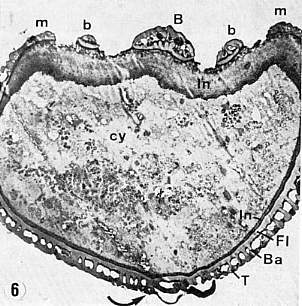
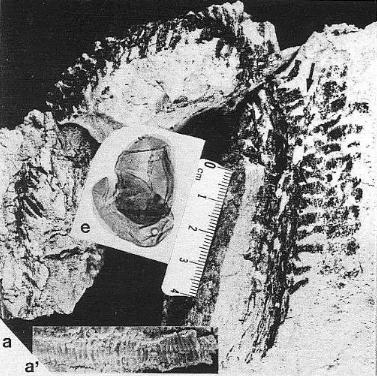
Part of the problem seems to be one of clarity or certainty that the fossil material was correctly interpreted. After all, the stakes are high. For example, Doyle and Donoghue (1993, p. 162) state, "Potentially most diagnostic are the female structures [of Sanmiguelia], interpreted as flowers with a perianth and carpels containing anatropous ovules, but we are not convinced that the preservation is clear enough to warrant Cornet's detailed reconstruction [see illustration below]." Judge for yourself if the most important fossil material is not clear enough (from Cornet, 1986; Cornet, 1989b):
At first glance the preservation of the carpel compression below may seem poor, but carpel and ovule cuticles are well preserved. The compression was fragmented before the rock was split, so that some pieces of compresson stayed with the part, and others clung to the counterpart, giving the specimen its fragmented appearance. This manner of preservation allowed pieces of carpel to be viewed in cross section, pieces of compression to be removed and examined with SEM, and pieces processed in oxidizing acids to separate and elucidate individual cuticle layers. Because of the fragmentation of the carpel, the surface impressions of both halves of the carpel are visible, allowing flaps of cuticle to be seen embedded in the rock matrix in places where the outer carpel cuticle was torn in distinctive arc-like cuts (arrows), typical of beetle bite marks. Pieces of anthers were found clinging to the outer cuticle, embedded in the adjacent rock matrix. The anther fragments were presumably brought there by herbivorous insects (probably beetles). Chewing insects would have had to visit male catkins first, then female flowers in search of food. In addition, dark-colored stains (g) can be seen in the matrix near the distal end of the carpel where glands for attracting insects were located. The location of two near basal anatropous ovules (o) could be determined based on a thicker compression containing multiple layers of cuticle, representing the various ovule integuments. Through compression examination and processing, even an internal tissue resembling pollen tube transmission tissue was discovered. Thus, the manner of preservation made possible an accurate determination of this organ's characteristics, proving that it is morphologically identical to an angiosperm carpel (see drawing B below). The isolation of this carpel is probably the result of insect herbivory.
|
bs = base; o = ovules; g = glands; s = stigmatic area; arrows = insect bite marks?
A flower receptacle showing mature follicles, some of which contain nearly mature seeds
sd = seed casts; three-dimensionally preserved.
A-C. Axelrodia burgeri (Sanmiguelia) female flowers and gynoecia. A. Carpel showing apical stigmatic area (top) from three-dimensionally preserved gynoecium in image above this figure (scale x 4; arrows denote carpel margin). B. Isolated carpel shown at same scale as flower in C, for comparison to help you identify overlapping carpels of gynoecium (gy) in C (scale x 2). C. Axelrodia burgeri apocarpous flower of the type terminating secondary inflorescence branch, showing four conduplicate bracts (cb1-cb4) subtending whorl of hairy bracts (hb), digitate bract (db), secondary branch (2br), and scale bract (sb); double-headed arrow shows base of cb1 emerging in a window through the gynoecium (scale in mm). The reproductive unit in C reveals an organization and morphology identical to that of an angiosperm flower (especially a differentiated perianth), and different from that known for the Gnetales and Bennettitales.
This misleading opinion (that Sanmiguelia is not preserved well enough to justify Cornet's detailed reconstruction) may be partly responsible for the omission of Sanmiguelia from most recent papers that discuss angiosperm evolution, despite the fact that molecular biologists such as Martin et al. (1993) accepted Cornet's description, calling Sanmiguelia an ancient angiosperm. Hochuli (pers. comm. 2005) did not know of my papers on Sanmiguelia when he published his manuscript on Ladinian angiosperm-like pollen (Hochuli and Feist-Burkhardt, 2004), and recent papers by Soltis et al. (2005) and De Bodt et al. (2005) make no reference to pre-Jurassic fossils of potential angiosperms, stating only that estimated ages for specific angiosperm clades using molecular estimates are generally older than inferences from the fossil record.
The new material on Sanmiguelia meets and in some cases surpasses the amount and quality of data used to identify most Cretaceous angiosperms. The paleobotany textbooks of Taylor and Taylor (1993: 727) and Stewart and Rothwell (1993: 380) provide important references to and illustrations of the new material.
Key angiosperm synapomorphies
(shared, derived characteristics that unite all angiosperms)
| Synapomorphies | Fossilization | |
| 1. Ovules are enclosed within an ovary, the outer wall of which is a carpel, which demonstrates a stigma where pollen germinates. | Carpel, stigma, ovary, and ovules can be and must be preserved in order to demonstrate these synapomorphies. | |
| 2. Double fertilization, which leads to endosperm formation (a nutritive tissue that feeds the embryo). | DF is impossible to demonstrate in the fossil record, although direct (petrified) and indirect (cast-mold) evidence for a nutritive tissue can be preserved. Pollen tubes have been found in silicified reproductive organs. | |
| 3. Stamens (microsporophylls) containing two pairs of pollen sacs (four pollen sacs in total). Pollen sacs lack a separate cuticle as in gymnosperms, and the septum that separates each pollen sac of a pair disappears with pollen development and maturity. | Microsporophylls containing two pairs of pollen sacs, a septum separating pollen sacs in each pair, and the absence of an outer pollen sac wall or cuticle can be demonstrated in very well preserved material. | |
4. Features of gametophyte structure and development (e.g. anatropous versus orthotropous embryos, one or two cotyledons, etc.). |
Diagnostic features of the gametophyte are sometimes preserved and evident in features of the seed. | |
| 5. Phloem tissue composed of sieve tubes and companion cells. | Except for well-preserved silicified plant stems, phloem tissue is rarely preserved or not preserved well enough to document these synapomorphies. |
See Doyle and Donoghue, 1986; Judd et al. 2002; P. Soltis et al., 2004; and D. Soltis et al., 2005, for further discussion. http://tolweb.org/tree?group=angiosperms
"All available evidence strongly rejects hypotheses of more than one evolutionary origin of extant angiosperms" (Soltis et al., 2005), meaning that angiosperm features are sufficiently distinct that evolutionary parallelism or convergence with non-angiosperms in most or all of these key synapomorphies is highly unlikely. Example: the Gnetales, which demonstrate a partial synapomorphy: double fertilization but without endosperm development (Friedman and Carmichael, 1996).
Sanmiguelia lewisii


Left: Leaf of Sanmiguelia from Brown (1956); right: Roland W. Brown (Courtesy, Henry N. Andrews and T.N. Taylor).
Brown (1956)
discovered, described, and named the first leaf impressions of Sanmiguelia from
the Late Triassic Dolores Formation of Colorado (outcrops along San Miguel
River). Only leaf impressions were discovered, with no organic material preserved in
the siltstone layers that entombed the fossils in growth position. At best, only two
vein orders are visible in the leaf impressions. Based on only leaf morphology,
Brown suggested Sanmiguelia might be a Triassic palm. That suggestion was
quickly dismissed by skeptics due to an absence of diagnostic leaf characteristics for
palms and an absence of reproductive organs, which are required to prove affinity or
taxonomic relationships.
 Bock (1969)
describes additional specimens of Sanmiguelia from Colorado, and classified this
plant as a member of the Cycadales. He devoted 22 pages to this fossil, claiming to
have recovered 150 specimens from the holotype locality. He suggested that Sanmiguelia
is a junior synonym of Paloreodoxites, a suggestion that has not been followed by
other paleobotanists. He even illustrates an impression fossil of Synangispadixis
next to a Sanmiguelia leaf, with accompanying line drawing, but missed its
significance. However, without well-preserved reproductive structures, the affinity
of this enigmatic plant remained controversial.
Bock (1969)
describes additional specimens of Sanmiguelia from Colorado, and classified this
plant as a member of the Cycadales. He devoted 22 pages to this fossil, claiming to
have recovered 150 specimens from the holotype locality. He suggested that Sanmiguelia
is a junior synonym of Paloreodoxites, a suggestion that has not been followed by
other paleobotanists. He even illustrates an impression fossil of Synangispadixis
next to a Sanmiguelia leaf, with accompanying line drawing, but missed its
significance. However, without well-preserved reproductive structures, the affinity
of this enigmatic plant remained controversial.
Left: Bock's 1969 reconstruction of Sanmiguelia, making it look like a cycad.
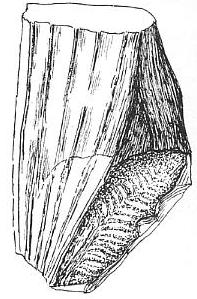

Above left: Figure 405 from Bock (1969) showing impressions of a Sanmiguelia leaf next to an "unidentified cone," which is identical in shape or morphology to Synangispadixis tidwellii from Sunday Canyon, TX, illustrated above right (a), from Cornet (1986). Synangispadixis is the male inflorescence of Sanmiguelia lewisii.
Becker (1972) describes globose to orbicular seed-like objects and possible fruits associated with the remains of Sanmiguelia lewisii from the type locality. The seed-like bodies described by Becker are similar to those of Nemececkigone fabaforma, the seeds of Sanmiguelia, and are of variable size: 7-12 mm in length by 5-10 mm in width. These seeds fall into the smaller size class for the Sunday Canyon material. They also show indications of wall striations, as well as a smooth seed coat.
Tidwell et al. (1977) recovered in situ specimens of stems that showed the manner of leaf attachment at Brown's holotype locality, including its herbaceous growth habit, but give no information on its reproductive structures. They compare Sanmiguelia to Veratrum spp., a genus in the Liliaceae. That was a bold comparison in the 1970s when the Cretaceous Angiosperm Origin and Primary Radiation Theory was gaining rapid acceptance among botanists and non-botanists around the world.



Left: Tidwell, Simper, and Thayn's reconstruction of Sanmiguelia; Center: Veratrum californicum; Right: W.D. Tidwell at 1991 AIBS meeting in San Antonio, TX.

Above: Sanmiguelia lewisii locality in Sunday Canyon, TX (upper Trujillo Fm., 1986).
 It wasn't until the
discovery of leaves with preserved carbonaceous material (Ash, 1976) that hope for better
preservation existed. Since the specimens reported by Ash came from a previously
unrecognized locality in Sunday Canyon, Texas (upper Trujillo Fm. of the Dockum Group),
and had indications of veins preserved between the plications, an attempt was made by
Cornet in 1980 to recover more complete material from Ash's locality. For three days
of excavation only fragments of leaves similar to those illustrated by Ash were found, but
persistence and luck eventually combined to uncover an interconnected colony of plants preserved in situ, with well-preserved
leaves possessing four orders of parallel veins, cross veins, and apical vein fusion
(distinctive monocot characteristics due to lateral growth from intercalary
meristems), stems, rhizomes, and stolons with roots attached, leaves and reproductive
structures attached to the stems, seeds, embryo casts, pollen, and petrified (silicified)
wood, roots, and ovules.
It wasn't until the
discovery of leaves with preserved carbonaceous material (Ash, 1976) that hope for better
preservation existed. Since the specimens reported by Ash came from a previously
unrecognized locality in Sunday Canyon, Texas (upper Trujillo Fm. of the Dockum Group),
and had indications of veins preserved between the plications, an attempt was made by
Cornet in 1980 to recover more complete material from Ash's locality. For three days
of excavation only fragments of leaves similar to those illustrated by Ash were found, but
persistence and luck eventually combined to uncover an interconnected colony of plants preserved in situ, with well-preserved
leaves possessing four orders of parallel veins, cross veins, and apical vein fusion
(distinctive monocot characteristics due to lateral growth from intercalary
meristems), stems, rhizomes, and stolons with roots attached, leaves and reproductive
structures attached to the stems, seeds, embryo casts, pollen, and petrified (silicified)
wood, roots, and ovules.
Above left: Sidney R. Ash during visit to
Cornet's residence in Houston in 1981.
Ash (1987) defines a Zone of Sanmiguelia in the Upper Triassic sequences of the Colorado Plateau, noting occurrences in the lower Owl Rock Member of the Chinle Formation, the lower Rock Point Member of the Wingate Sandstone (which overlies the Chinle Fm.) in northeastern Arizona, and the middle member of the Dolores Formation in southwestern Colorado. He gives an age range for the Zone of Sanmiguelia as Norian-Rhaetian. He notes that Sanmiguelia is commonly associated with Pelourdea poleoensis in the Owl Rock Member and the Dolores Fm. (holotype locality for Sanmiguelia near Placerville, CO). Both plants occur together in the uppermost Trujillo Formation of the Dockum Group of northern Texas (the Sunday Canyon locality reported by Ash, 1976, and Cornet, 1986).
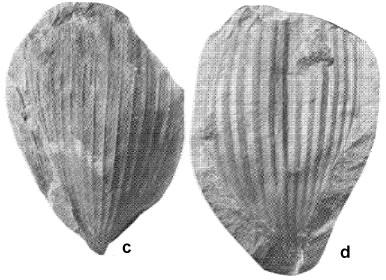
Above: Sanmiguelia lewisii leaves collected by Ash (1987) from the type locality in the Dolores Formation, southwestern Colorado.
When Ash was asked what he thought the affinity of this plant was before Cornet's 1986 paper, he said he thought it was a cycad, similar to Bock's interpretation and reconstruction of it. As a cycad, it would be an unremarkable plant for the Triassic, and not worthy of further consideration as an angiosperm or angiosperm ancestor.
Aerial Parts

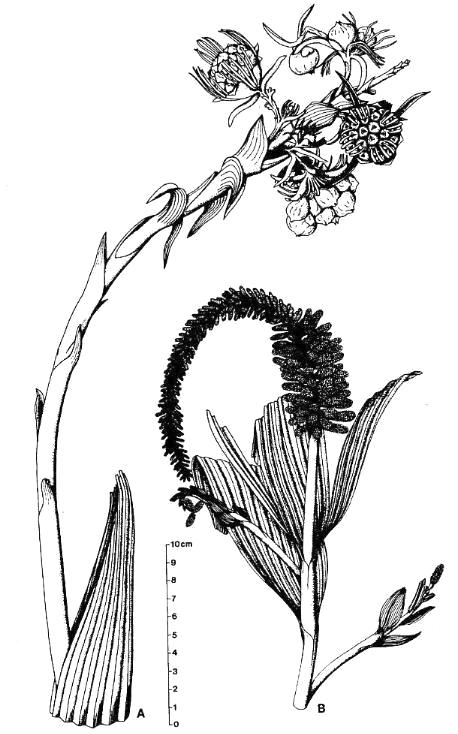
Above left: Cornet's 1986 reconstruction of Sanmiguelia, revealing its resemblance to monocots and dicots. These drawings are based on actual specimens without any significant interpolation of missing or concealed structures (actual images of fossils to right and below). Because the Sunday Canyon specimens were not found attached to the same individual, Axelrodia burgeri (A) and Synangispadixis tidwellii (B) were created by Cornet (1986) as names for the female and male inflorescence of Sanmiguelia lewisii, respectively. These specimens demonstrate that Sanmiguelia is neither a cycad nor a gnetalean.
Subterranean Parts
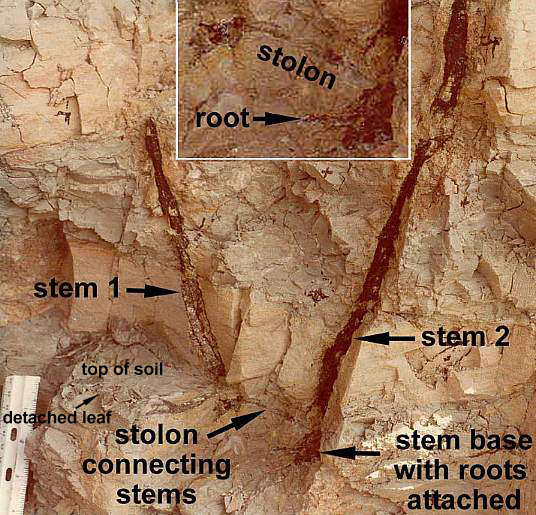
Above: Sanmiguelia was found preserved in growth position; a vegetative colony connected by rhizomes and stolons to roots, which were traced through the paleosol. Secondary wood was preserved in the lower parts of vertical axes. Rarely do paleobotanists recover so many parts of one plant, making this fossil an extraordinary find. For more information on growth habit, go to The Stem and Root Anatomy of Sanmiguelia lewisii, and a Comparison with Extant Dicots and Monocots.
Below: Angiospermous characteristics of Sanmiguelia lewisii (from Cornet, 1986; Cornet, 1989b). Go to the linked papers for details and supporting evidence not given here. Sanmiguelia fossils demonstrate three of four groups of preservable key synapomorphies (above in bold green).
* A closed ascidiate (barrel-shaped) carpel with ventral suture.
Above left: Map of isolated carpel compression; above right: Reconstruction from Cornet (1989b). Locations for slide preparations (SLD) and scanning electron micrographs (SEM) of carpel compression are shown.
Above left: Thin inner carpel cuticle showing stomata (Cornet, 1989b). Above right: Thicker outer carpel cuticle showing numerous hair bases and one stomata (upper right).
* A specialized area for pollen germination - an apical stigmatic area.
* An ovarian organ similar to that adapted in angiosperms for pollen-tube transmission.
Above left: SEM view of middle tissue layer inside carpel compression (between inner carpel cuticles), showing alignment of papillae, which would help enable and direct pollen tubes to find ovules. Above right: TL image of middle layer, showing similar alignment of surface structures (Cornet, 1989b).
* Paired near-basal anatropous ovules with indications of two integuments (meaning that ovules could not have been pollinated directly as in gymnosperms). We know this because of seed shape (right- and left-handed forms), two nearly mature seeds found within follicle (see above), and two ovule compressions found in several carpels (see above).
* Statistical data on organ growth indicating that anatropous ovules were remotely pollinated and developed after fertilization (i.e., well after carpel development and closure).
* A dicotyledonous embryo, one cotyledon of which was larger than the other cotyledon (accounting in part for the asymmetrical shape of seeds). Embryo cast below. See reconstruction in figure b.
Scale in mm.
* Large lima bean-shaped seeds with smooth seed coat (Nemececkigone fabaforma), radicle, hypocotyl, and plumule, all of which have recognizable features in an embryo cast (See photograph and figure b below). Micropylar scar next to hilum scar indicates anatropous condition. Seed coat split longitudinally in half ('i' below).
* Monoecious with separate male (Synangispadixis) and female (Axelrodia) reproductive axes. The presence of unisexual flowers is a response in angiosperms to the loss of a self-incompatibility system and the presence of imperforate psilate monosulcate pollen.
* Ovuliferous flower-like units possessing a perianth comprised of long and specialized bracts. The digitate lobes of petals each have a single supporting vascular strand; they resembles those of Illicium floridanum (Schisandraceae sensu APG II (2003).
Above: Axelrodia burgeri female flower with isolated carpel (left) for comparison. See labeled diagram above and Cornet (1989b).
Above: Flower of Illicium floridanum for comparison of petal lobes.
* Naked polliniferous flower-like subunits organized into catkin-like branches, comparable to that of Hedyosmum mexicana: Chloranthaceae, which are placed by molecular results between the ANITA group and Eudicots (See cladogram below).
Above: Synangispadixis tidwellii showing secondary branches bearing immature, naked spirally-arranged unistaminate flowers, similar to those of Hedyosmum mexicana (not illustrated in Cornet, 1986 or 1989b).
* Fused pollen sacs borne in pairs (= tetraloculate anther) and lacking distinct pollen sac walls (a key angiosperm synapomorphy). Connective reduced so that paired pollen sacs are joined only at their base. Bilaterally symmetrical pollen sacs oriented in pairs such that the septa are aligned in same plane. Pollen sacs elongated away from secondary axis as they matured, similar to those of Hedyosmum (illustrated in figure above).
Above: Selected and modified from Cornet, 1986 and 1989b; immature microsporophylls joined at base in pairs; t = pointed tip, b = base.
* Anther septa disappear at pollen maturity (a key angiosperm synapomorphy - proves Sanmiguelia is not gnetalean or cycadalean).
Figure 4. a-c. Line drawings from SEMGs of microsporophylls to Synangispadixis tidwellii Cornet sp. nov. PP34322. "Mummified" microsporophylls broken open to reveal two pollen masses (cross hatched) of different size (1 larger than 2), separated by a septum (SE) of variable development, thick-walled "fiber" cells (FB) adjacent to the suture, probable epidermal (EP) and endothecial (EN) cell layers near base (BS), and outlines of individual pollen grains; photograph of specimen a. in Pl. 7, fig. b; bar scales 100 um long. Note outer pieces of wall removed to reveal internal structure. From Cornet (1986). A septum divides the two pollen masses when the pollen grains are immature, and disappears in anthers containing mature pollen - a unique angiosperm characteristic.
* Monosulcate pollen similar to that of derived Magnoliid angiosperms that have lost self-incompatibility, and consistent with the Late Triassic trend in angiosperm-like pollen towards pollen with a single distal aperture and reduced tectate-granular exine structure.
Above: Sanmiguelia pollen (stained) clinging to anther cuticle; note faint granular wall structure.* Variable number of carpels per flower, ranging from one to nearly a dozen per gynoecium, similar to the variability found in Amborella and other members of the ANITA group. Flowers with many carpels found at ends of secondary branches, while flowers with single carpels attached along secondary axes (see reconstruction below).
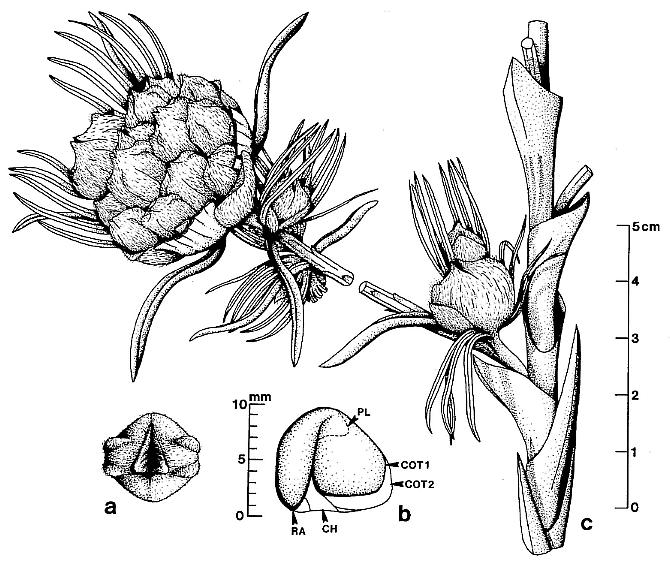
Above: From Cornet, 1986 (Evolutionary Theory: Fig. 7). Embryo reconstruction in 'b' based on a cast found inside Nemececkigone fabaforma. The shape of the embryo is supported by the external shape of the seed.
Above: Schematic drawings of floral morphology (Cornet, 1989b; on cover of Evolutionary Trends in Plants).
* Secondary vesselless wood present only in the basal portion of main stems (up to 30 cm above paleosol); secondary wood possessed circular bordered pits, uniseriate and multiseriate rays, and circular leaf and branch traces in wide multilacunar leaf gaps (demonstrated in both petrified wood and in pith casts: see below). In the upper part of vertical stems the pith cast lacks evidence of secondary growth and shows very wide and elongate vascular gaps spirally arranged around a wide pith.

Pith cast on left not retouched; same pith cast on right shows multilacunar rays highlighted in white between lenticular leaf gaps.
* Long elliptical leaves with four orders (widths) of parallel veins, several types of cross veins, and apical vein fusion; actinocytic to anomocytic stomata; scalariform (dominant), reticulate (common), and helical (rare) tracheids in veins; common resin cells (aligned and containing resin) that form elongate trains and follow veins. Primary veins separated mainly by tertiary and quaternary veins, with secondary veins arising from division of primaries. Secondary veins 0.4 mm-0.99 mm in width. Tertiary veins 0.1 mm-0.39 mm in width. Quaternary veins less than 0.1 mm in width down to a single strand of tracheids. Primary, secondary, and tertiary veins composed mainly of scalariform tracheids; tertiary and quaternary veins composed of scalariform and reticulate tracheids; quaternary veins sometimes composed of single or double strands of annular-helical tracheids. Epidermis thin, moderately covered with single hairs and dendroid trichomes. No extant or fossil gymnosperm leaf possesses this combination of features; comparison can be made only with angiosperm leaves (mainly monocots).
Left: Apical portion of a well-preserved Sanmiguelia leaf showing progressive anastomosing of veins going into the leaf tip. Right: Central portion of a well-preserved leaf showing secondary veins located mostly in the folds of plications.
Portion of Leaf: BASE LOWER MIDDLE UPPER TIP Vein Density (per mm width): 1.76-1.82 2.1* 2.4 2.2-2.9 3.4 Quaternaries: 44-49% 26% 62% 54-86% 41% Tertiaries: 33-35% 66% 32% 44-12% 32% Secondaries: 20-10% 8% 6% 2% 27% Primaries 3-6% -0- -0- -0- -0- Above table shows distribution of vein types (based on width) from the base to tip of Sanmiguelia leaves (from Cornet, 1986). Note how secondary and tertiary veins increase in number (through anastomosis) going into the leaf tip.
Above: Peel of a portion of leaf from near its base, showing one primary and several secondary veins dividing upwards into expanded portion of leaf lamina.
Above left: Basal portion of a leaf showing secondary veins, some of which are connected by cross veins. Above right: Two tertiary veins with scalariform and reticulate tracheids, interconnected by a cross vein. Numerous actinocytic stomata in the cuticle can be seen between veins. Natural color (unstained).
Above left: Secondary, tertiary, and quaternary veins showing cross veins, one of which (center) diverges from a secondary vein (left), crosses another secondary vein, and joins with a third secondary vein (center). Above right: a quaternary vein showing scalariform and helical tracheids. Natural color (unstained).
Above left: Lower portion of a leaf showing secondary, tertiary, and quaternary veins, and a wide resin canal with dark red resin still in place. Above right: A cross vein connecting two tertiary veins, and a small resin canal (right). Natural color (unstained).
Above left: A portion of leaf cuticle between veins, showing scattered simple epidermal hairs (darker than epidermal cell outlines). Above right: A branched trichome (tc) next to a simple hair (hr).
* Vessels in the roots; tracheids variable in length and width, up to the size of small vessels; had crowded simple side-wall pitting and scalariform end-wall pitting, similar to what occurs in the aquatic water lilies.
Note large range in size of tracheids in silicified root of Sanmiguelia. Natural color (unstained).
 There is nothing
about this plant which says it could not be an angiosperm, albeit a very primitive one;
dicot-like stems with secondary wood and multiseriate rays (common among herbaceous
dicots); long angiosperm-like inflorescences with flower-like units that were not yet
genetically stable or regular (an ideal pre-flower condition that could evolve into
various floral plans); a dicotyledonous embryo intermediate between a dicot and monocot
condition (consistent with it having monocot-like leaves and a woody stem); an herbaceous
semiaquatic habit (this plant vegetatively resembled extant Veratrum californicum, Liliaceae:
Tidwell et al., 1977) at the edge of a small lake or floodplain pond where it
lived among ferns and other herbaceous seed plants (this is the same type of habitat from
which Cretaceous angiosperms began their radiation (Hickey and Doyle, 1977; cf. Nelumbo
and Archaefructus).
There is nothing
about this plant which says it could not be an angiosperm, albeit a very primitive one;
dicot-like stems with secondary wood and multiseriate rays (common among herbaceous
dicots); long angiosperm-like inflorescences with flower-like units that were not yet
genetically stable or regular (an ideal pre-flower condition that could evolve into
various floral plans); a dicotyledonous embryo intermediate between a dicot and monocot
condition (consistent with it having monocot-like leaves and a woody stem); an herbaceous
semiaquatic habit (this plant vegetatively resembled extant Veratrum californicum, Liliaceae:
Tidwell et al., 1977) at the edge of a small lake or floodplain pond where it
lived among ferns and other herbaceous seed plants (this is the same type of habitat from
which Cretaceous angiosperms began their radiation (Hickey and Doyle, 1977; cf. Nelumbo
and Archaefructus).
Above left: Veratrum californicum (courtesy W.D. Tidwell).
Sanmiguelia |
Nelumbo |
| Herbaceous Semiaquatic Dicotyledonous embryo Large apocarpous flower terminating inflorescence Multiple rings of carpels Secondary xylem in stems and roots Vessels in roots |
Herbaceous Aquatic Dicotyledonous embryo Large apocarpous flower terminating inflorescence Multiple rings of carpels Secondary xylem in stems and roots Vessels in roots |
Molecular research has been a major force in the re-evaluation of angiosperm taxonomy, long overdue, and has resulted in the overturning of many previously held beliefs, such as the Magnoliales containing basal angiosperms, the Nymphaeales being linked to monocots, and the grasses being derived from monocots (as opposed to being a separate monophyletic lineage based on some molecular data). Doyle and Endress (2000) and Doyle (2001) explore these new taxonomic relationships, creating a composite cladogram using morphological and molecular data. This new taxonomy can be tested using the fossil record. Two areas of considerable interest are the time of evolution of tectate-reticulate-columellate pollen and time of evolution of the multilacunar node [for an important discussion on the evolutionary significance of Sanmiguelia stem anatomy, see The Multilacunar Node of Sanmiguelia lewisii]. If these two events occurred within the crown-group, as Doyle and Endress (2000) propose, then pre-Cretaceous evidence for the existence of these characteristics would support the older age estimates for angiosperms (200-230 mybp) based on molecular data (Sanderson and Doyle, 2001). When the data for the Crinopolles Group and Sanmiguelia are plotted on their composite cladogram for carpel form (their Fig. 7), the nature of the controversy over when angiosperms evolved takes on stunning clarity: Is the Cretaceous radiation of angiosperms related to the origin of the crown-group, and can stem-group angiophytes be called angiosperms? For example, is Nuphar with its diploid endosperm and near basal position on the new cladogram a stem-group angiosperm or a crown-group angiosperm? And what about Amborella?
Composite cladogram showing the distribution of Sanmiguelia characteristics.
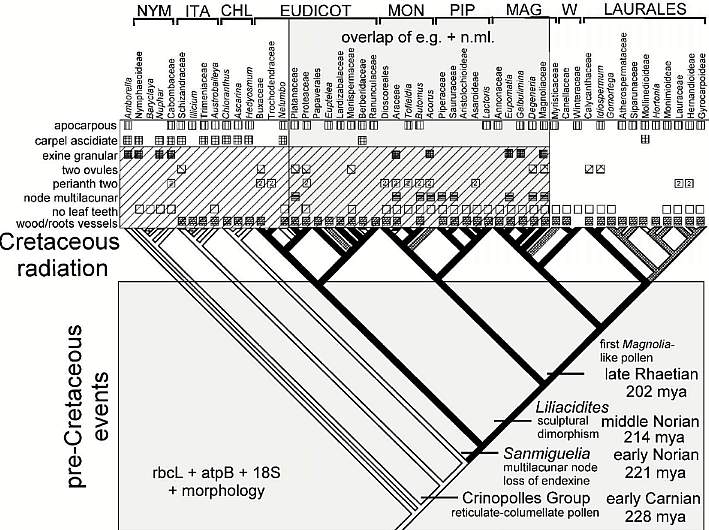
Modified after Fig. 7, Doyle and Endress (2000). NYM = Nymphaeales; ITA = named after Illicium, Trimeniaceae, and Austrobaileya; CHL = Chloranthaceae; MON = Monocotyledonae; PIP = Piperales; MAG = Magnoliales; W = Winterales.
And finally, the
problem paleobotanists are having with Sanmiguelia can be put into perspective:
By the above analysis, Sanmiguelia is more derived than Amborella, NYM,
ITA, and perhaps CHL. Nelumbo is not much more derived than Sanmiguelia
(compared to all angiosperms). Most of the character states identified by Cornet for
Sanmiguelia are widely distributed among angiosperms, as would be expected if
they are pleisiomorphic (i.e., not derived). The ascidiate carpel (with a pair of anatropous
ovules), granular exine, and multilacunar
node, however, are of diagnostic value. The ascidiate carpel is characteristic
of pre-Eudicots, while the granular exine is characteristic of basal angiosperms, some
monocots, and the Magnoliidae. In other words, the granular exine may be a
pre-Crinopolles characteristic, but it has been retained or resurrected (reversal) in
post-Sanmiguelia angiosperms. The Hedyosmum-like male
inflorescence may indicate a post-ANITA group (even a post-Chloranthaceae) placement in
Doyle & Endress's composite cladogram (above), because that
type of reduced male flower does not occur below the Chloranthaceae. Their cladogram
also indicates that the multilacunar node plus leaves with parallel venation, etc., are
derived characteristics within the crown-group. That means Sanmiguelia
may belong on the branch leading to monocots, and is therefore a bona fide
angiosperm.
Bill Burger (left) and Bruce Cornet (right, AIBS, University of Ohio, 1987); Cornet gave a talk on Sanmiguelia at this convention.
If Sanmiguelia and Crinopolles pollen belong to pre-angiosperms (i.e., the pre-Cretaceous events in Fig. 7 took place in stem-group angiophytes), the crown-group would be polyphyletic, and angiosperms which still produce diploid endosperm may not belong in the crown-group. And if Sanmiguelia and Crinopolles pollen are not related to angiosperms at all, how will paleobotanists recognize a stem-group angiophyte or pre-angiosperm now that the Gnetales and all other extant gymnosperms have been excluded?
The frequency of parallel-veined leaves among Mesozoic seed plants (e.g. Pelourdea) needs to be compared with the number of primitive pollen morphotypes found in monocots (see The Significance of Polyplicate Pollen), possibly indicating that botanists should reconsider Burger's 1981 thesis: Heresy Revised: the monocot theory of angiosperm origin.
More Evidence for Late Triassic Angiosperms
Parallel-veined leaves like those of Pelourdea and Sanmiguelia have been found at the Delevoryas and Hope (1973) fossil plant locality (Boren Clay Products Co. pit) in the late Carnian Pekin Formation of the Deep River basin, North Carolina, U.S.A. Unfortunately, the Delevoryas and Hope plant bed has been mined out. Other facies lenses containing plants await future discovery.


Above left: Ted Delevoryas at OSU paleobotany convention, 1987; above right: Bob Hope at his fossil plant collection barn in Clinton, NC, 1989.
That locality has produced numerous megafossil compressions of ferns (e.g. Cladophlebis, Phlebopteris, Cynepteris), sphenophytes (e.g. Neocalamites), cycads (e.g. Leptocycas), cycadeoids (e.g. Otozamites, Pterophyllum, Pseudoctenis, Williamsonia), and conifers (e.g. Florinostrobus, Compsostrobus) (Delevoryas, 1970; Delevoryas and Hope, 1973; 1975; 1981). Monocrinopollis microreticulatus pollen was illustrated by Schultz and Hope (1973) from that locality. It is the youngest and most derived Crinopolles pollen type from the Richmond basin of Virginia (Cornet, 1989a).


Above left: Otozamites powellii; above right: Basal portion of a tree fern frond showing large Cyclopteris-like stipules at the bases of secondary branches. Both specimens from the Bob Hope fossil plant collection (photographed by Cornet in 1989).



Above left: Compsostrobus conifer foliage; above center: Compsostrobus cone; above right: Neocalamites sp. (Equisetaceae). Compsostrobus is thought to be the ancestor of the Pinaceae, prior to the evolution of short shoots and fascicles of needle-shaped leaves. Its seed cone is remarkably similar in morphology to that of the Pinaceae, although longer and narrower.


Above left: Millerostrobus, the male cones of Compsostrobus; above right: Williamsonia "flower", which probably came from a cycadeoid bearing Pterophyllum leaves.


Above left: Leptocycas frond (Cycadales); above right: Pterophyllum frond (Bennettitales).

Above center: Eoginkgoites frond; upper left: Tongue-shaped Podozamites leaf; top center: Compsostrobus foliage. All of the gymnospermous leaves from the Delevoryas and Hope locality have parallel venation, but none has a sheathing leaf base nor apical vein fusion.

Above: Phlebopteris sp., a dipteridaceous fern.
In 1973 the author discovered at this locality an herbaceous plant with lanceolate-shaped leaves, plications, a central midvein, at least two orders of parallel veins, and apical vein fusion.




It has not yet been formally named. It was discovered in a dark gray claystone near the base of the exposure, and has stems attached to leaves, associated with a central root system with secondary and tertiary roots attached (like that of Sanmiguelia). In addition, a single angiosperm-like stamen was found in the same leaf bed, which demonstrates an anther-like structure with connective and a filament that extended below and above that structure (see below). Because only one specimen was found, it was not damaged by sampling to recover pollen. The specimens illustrated below are currently in the possession of Brian Axsmith at the University of Southern Alabama. Axsmith also has the plant collection of Hope.

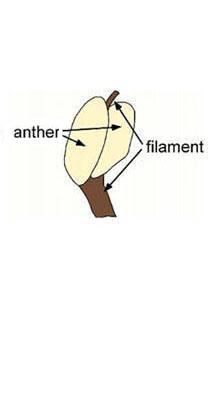
Above: Angiosperm-like stamen with connective extending below and a filamentous extension extending above paired anther-like structures. Below the angiosperm-like stamen is a leaf similar to those illustrated below. Scale in mm.
The leaves have very long sheathing leaf bases, similar to those of Sanmiguelia. The stems appear to have had a wide central pith, because the stems are collapsed, indicating the absence of a woody cylinder. The presence of a 'midvein' and central fold or plication distinguishes this fossil from both Sanmiguelia and Pelourdea. The 'midvein' is sometimes difficult to distinguish; it is only slightly wider than the lateral veins. Compare the median bundle of extant monocot leaves (see diagrams below). Some paleobotanists think that a central midvein is required for a monocot-like fossil plant to be an angiosperm, but most monocots do not have a central leaf midvein (Arber, 1925; e.g. Veratrum: Liliaceae, which Tidwell et al., 1977, compared with Sanmiguelia). In some cases, monocot leaves have bifacial venation (abaxial and adaxial sides of the leaf blade), such as in Thalictrum and Asphodelus (Arber, 1925: 114). In other words, the monocot leaf is derived from a ligular sheath. An apical spine or 'cornet' is sometimes the only remnant of the petiole, which is homologous with the petiole of dicot leaves. If the dicot blade was lost through reduction, there would be no need for a primary or central midvein. Therefore, the presence of a midvein in monocot leaves would be pleisiomorphic, unless it evolved secondarily, while an absence of a midvein would be apomorphic.
"The spine is not a mere emergence, but is the only part of the leaf which receives its vascular supply directly from the axis (figs. lxxxix, 2 and 3); the upper scale-like region of the leaf is entered exclusively by branches (l.b.), arising from the spine-bundle (s.b.). These facts seem to me to support the view, first suggested by Buscalioni1, that the scale is a ligular sheath, while the spine is the petiolar limb."
"We thus take a leaf-base terminating in a simple petiole as the fundamental Monocotyledonous type, and regard leaves such as those of Hemerocallis as representing a further degree of reducution, in which the petiole is altogether lost." (Arber, 1925: 115-117)
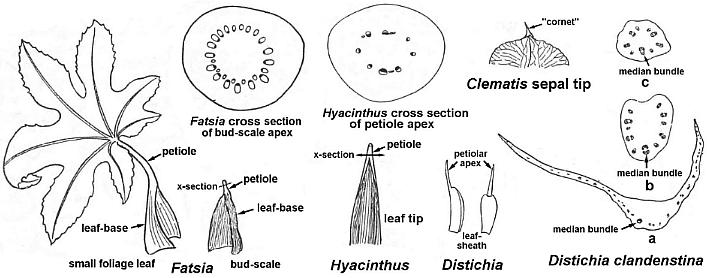
Above: Illustrations selected and modified from Arber (1925: lxxxvii and lxxxviii).
 Thus, the absence of
a strong central or primary vein is characteristic of most monocot leaves, and is of no
diagnostic value in determining affinity. The argument that the earliest monocots or
their immediate dicot ancestors should have a central midvein is not supported by the
evidence. The presence of gymnosperms and non-angiosperms with parallel venation in
the Triassic (e.g. Pelourdea, Podozamites, Czekanowskia, etc.)
cannot be used to argue that these monocot-like leaves are pre-angiospermous if all these
plants went through leaf reduction forced by environmental factors at or near the
Permo-Triassic boundary. In other words, Early Mesozoic seed plants with parallel
venation may represent convergent evolution. Parallel venation by itself does not
necessarily indicate affinity or relationship.
Thus, the absence of
a strong central or primary vein is characteristic of most monocot leaves, and is of no
diagnostic value in determining affinity. The argument that the earliest monocots or
their immediate dicot ancestors should have a central midvein is not supported by the
evidence. The presence of gymnosperms and non-angiosperms with parallel venation in
the Triassic (e.g. Pelourdea, Podozamites, Czekanowskia, etc.)
cannot be used to argue that these monocot-like leaves are pre-angiospermous if all these
plants went through leaf reduction forced by environmental factors at or near the
Permo-Triassic boundary. In other words, Early Mesozoic seed plants with parallel
venation may represent convergent evolution. Parallel venation by itself does not
necessarily indicate affinity or relationship.
The loss of a midvein in Sanmiguelia, combined with the presence of a possible midvein (undergoing reduction) in the new plant, imply that leaf reduction could have happened very early in angiosperm evolution. That does not mean that monocots evolved very early, only that the dicot branch leading to monocots evolved earlier than the latest phylogenetic trees indicate (explaining why there has been conflict with molecular data, some of which indicate an age older than Jurassic for monocots). This plant is older than Sanmiguelia (late Carnian versus Norian), which could indicate that diversity among early dicots with monocot-like leaves was considerably greater than paleobotanists have imagined. Today the monocots are the only surviving but derived members of that diversity.
Above left: Podozamites from the Delevoryas and Hope locality.

Above: Four lanceolate leaves on a single bedding plane, showing a central midvein (arrows) and plications that are well developed in the leaf tip. The 'midvein' occupies a fold, and is one vein slightly wider than the lateral veins.

Above: Leaf tip showing a central 'midvein' (arrows) flanked by numerous parallel veins.

Above: Stem with a sheathing leaf base diverging to the right.

Above: Primary root system preserved in situ on underside of a specimen containing the leaves and stems.
A Mid-Jurassic Angiosperm? with Monocot-like Leaves

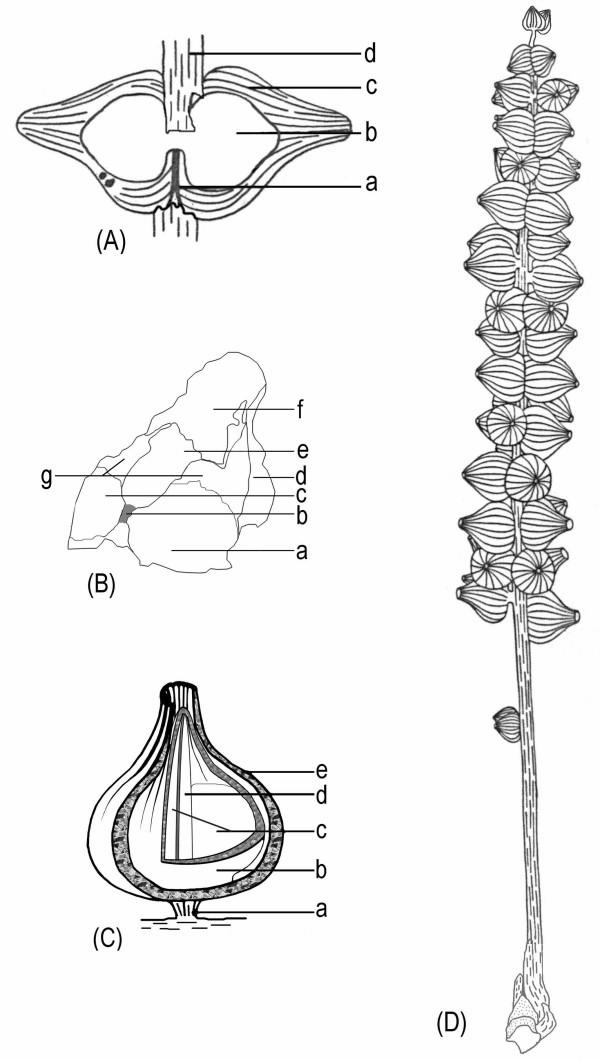
Above right: Diagrams of the female organs and the reconstructions of S. sinensis. a. Schematic diagram of the female organ pair shown in Fig. 2e. Note the female organs' fused bases (a), the central unit (b), the sheathing envelope (c), and the axis of the female structure (d). b. Schematic diagram of the female organ in Fig. 2g. Note the smooth locule walls (a and c, probably due to the fallen ovules) and the rough internal walls (g) on each side of the septum (b), different parts of the envelope (d and f), the relic of the broken central unit (e), the vertical septum (b) connected to the base of the central unit. c. A reconstruction of a female organ of S. sinensis. Note the short peduncle of the female organ pair (a) connected to the axis of the female structure (for simplicity, only one organ of the female organ pair is shown here), the central unit (b), two locules (c), the septum (d) separating the two locules, and the sheathing envelope (e). d. Schematic diagram of S. sinensis. From the bottom, note the apex of the short shoot, axis of the female structure, isolated immature female organ pair, clustered female organ pairs along the axis of the female structure, and terminal female organ pair. (used with permission from Xin Wang, 3/07) Note that the "carpels" of Schmeissneria are pedicellate, like those of the Carnian Primaraucaria (see root parasites below).
Above left: A general view of female structures, leaf, and short shoot. a. A general view of two female structures (a, b) and one leaf (c) on the same slab. [only
a. is shown] The specimen A is the holotype, and specimens B and C are the paratypes. Specimen numbers 8604a, 8604b, and 8604c. Bar = 2 cm. (used with permission from Xin Wang, 3/07)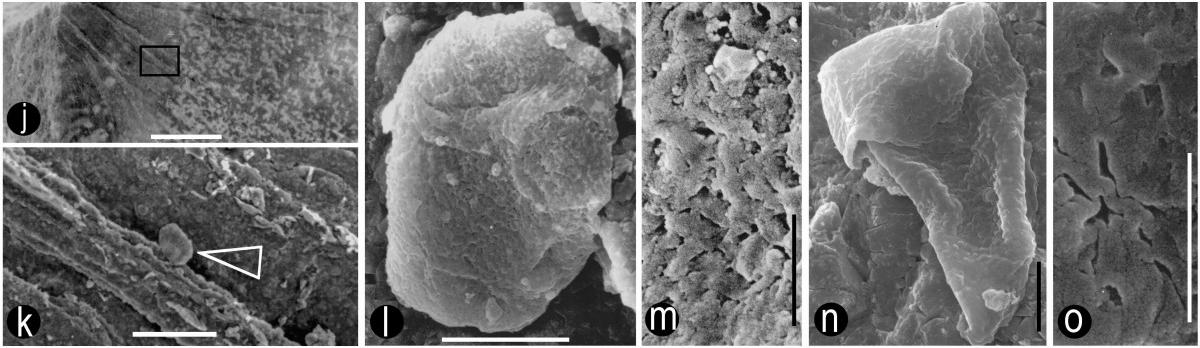
The pollen of Schmeissneria appears to be weakly monosulcate or inaperturate, similar to that of Amborella pollen. According to Doyle's (2005) analysis of basal angiosperm pollen, the presence of a rugulate (perforate) tectum, supratectal sculpture, and elliptical to globose shape agree with the types of pollen found in the ANHITA group.
Schmeissneria: A missing
link to angiosperms?
BMC Evolutionary Biology 2007, 7:14
"Abstract
"Background
"The origin of angiosperms has been under debate since the time of Darwin. While there has been much speculation in past decades about pre-Cretaceous angiosperms, including Archaefructus, these reports are controversial. The earliest reliable fossil record of angiosperms remains restricted to the Cretaceous, even though recent molecular phylogenetic studies suggest an origin for angiosperms much earlier than the current fossil record.
"Results
"In this paper, after careful SEM and light microscopic work, we report fossils with angiospermous traits of the Jurassic age. The fossils were collected from the Haifanggou Formation (middle Jurassic) in western Liaoning, northeast China. They include two female structures and an associated leaf on the same slab. One of the female structures is physically connected to the apex of a short shoot. The female organs are borne in pairs on short peduncles that are arranged along the axis of the female structure. Each of the female organs has a central unit that is surrounded by an envelope with characteristic longitudinal ribs. Each central unit has two locules completely separated by a vertical septum. The apex of the central unit is completely closed. The general morphology places these fossils into the scope of Schmeissneria, an early Jurassic genus that was previously attributed to Ginkgoales.
"Conclusion
"Because the closed carpel is a character only found in angiosperms, the closed apex of the central unit suggests the presence of angiospermy in Schmeissneria. This angiospermous trait implies either a Jurassic angiosperm or a new seed plant group parallel to angiosperms and other known seed plants. As an angiosperm, the Liassic age (earliest Jurassic) of Schmeissneria microstachys would suggest an origin of angiosperms during the Triassic. Although still uncertain, this could have a great impact on our perspective of the history, diversity and systematics of seed plants and angiosperms."
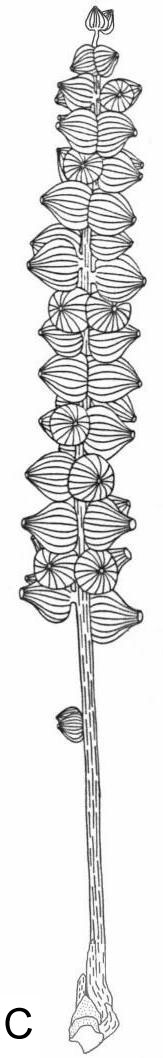
Comparison of Schmeissneria sinensis and Spathiphyllum clevelandii (Araceae). Are these structures homologous? The gynoecia of Schmeissneria have two locules each (they are also pedicellate and paired), while the gynoecia of Spathiphyllum have three locules each. The gynoecia of Anthurium have two or three imperfect locules each.
Schmeissneria from early Late Triassic? to Middle Jurassic
A Schmeissneria-like reproductive axis was discovered in the same Late Carnian (Late Triassic) strata that produce Pannaulika triassica, a dicot-like leaf. What is important about Schmeissneria is that it was originally assigned to the Ginkgoales because of its parallel-veined leaves; it may represent a basal group of angiosperms, here called the Schmeissneriales. In the diagrams below, where does the transition to angiospermy occur?
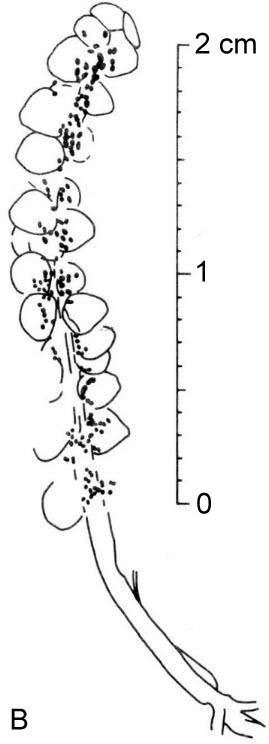

Late Triassic
Late Triassic
Early-Middle
Jurassic Late
Jurassic - Early Cretaceous
Strap-shaped parallel-veined leaves
Strap-shaped parallel-veined leaves
Note similar bauplan: A is the early Norian (Late Triassic) Archaestrobilus prior to cupule closure; B is a late Carnian (Late Triassic) reproductive axis from the Solite Quarry, NC/VA. C is the Early-Middle Jurassic Schmeissneria, used with permission from Xin Wang; it is at least twice as long as the Triassic example in B. D on right is the Late Jurassic - Early Cretaceous Archaefructus, showing a similar naked construction of the reproductive axis, i.e. without perianth parts (modified from Sun et al., 2002). The carpels of Archaefructus are also paired as they are in Schmeissneria, providing a possible evolutionary link between them.
So, how does Amborella with its reticulate-veined pinnate leaves fit into early angiosperm evolution, if the majority of earliest angiosperms possessed parallel-veined leaves that have been confused with those of gymnosperms? The reticulate-veined pinnate-palmate leaves of Pannaulika triassica (see below), if they do not belong to a dipteridaceous fern, may provide a partial answer. Such leaves would show that early angiosperms were capable of producing a wide variety of leaf types.
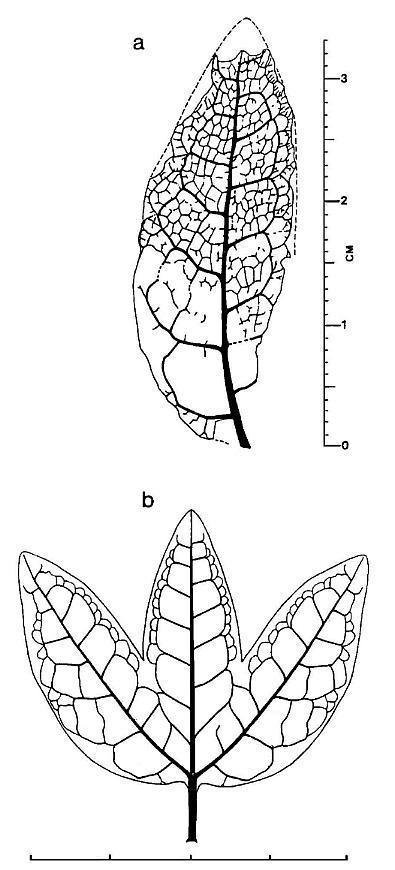
Scale in cm.
A Living 'Dicot' with Monocot-like Leaves!
The ANITA Group consists of Amborella, the Nymphaeales; Illicium, the Trimeniaceae, and Austrobaileya. These are considered to be the most primitive living angiosperms based on molecular (genetic) analysis (see Friis and Crane, 2007).
John Barrett (pers. comm. 12/2006) writes: "Hydatellaceae inconspicuous semi-aquatic plants of Austalia, New Zealand, India were until very recently considered MONOCOTS, but first MOLECULAR DATA and on re-examination MORPHOLOGY indicate they are a very basal SISTER of NYMPHEALES -- so I propose to add them to ANITAs and make them ANHITAS. I tell people that ANHITA in Neandertal mythology was the ancient mother goddess of all the flowers.
"Peter Stevens is waiting for formal publication of one of the Hydatellaceae papers before he shows the new information on the MOBOT website, but he plans to do so. Jim Doyle and most others support the change. During his trip to Europe this summer Jim spoke with Behnke who did so much work on sieve tube plastids - it would be interesting if someone would check the sieve tube plastids of this family Hyadellaceae - Behnke is alive but relatively inactive, and no one seems to be continuing his work - most major groups have been done, but it might be interesting to check Hydatellaceae." (Barrett: DCBR7@yahoo.com).
Family Hydatellaceae
Hydatella filamentosa from Dove Lake, Tasmania. Photograph © Botanic Gardens Trust, Sydney.
Photographer: Simone Cottrell. Used with permission.
Trithuria submersa from Western Australia (use permission given by Dennis Stevenson, NYBG, 3/07)
Hydatellaceae: Full family description from DELTA data Hydatellaceae: List of genera from the Royal Botanic Gardens, Kew Hydatellaceae: A family overview ...
www.csdl.tamu.edu/FLORA/cgi/gateway_family?fam=Hydatellaceae - 2k - Images:
- Embryo drawing (Trithuria submersa): U.S. National Seed Herbarium image
- Fruit (Hydatella filamentosa): U.S. National Seed Herbarium image
- Seeds (Hydatella filamentosa): U.S. National Seed Herbarium image
- Hydetallaceae (Hydatella and Trithuria): UBC Botanical Garden
This discovery is significant in that it shows that some early
angiosperms had monocot-like leaves, but were not monocots.
The ANHITA classification below is
modified from that of K. Kinman (pers. comm. 2007).
"... does the seemingly simple floral morphology of the Hydatellaceae reflect ‘reduction’, or might it represent an early ‘prefloral’ stage of angiosperm evolution in which the classic bisexual flower, with its whorls of three or more different organs, was not yet fully formed? Flowers of the Hydatellaceae - along with those of other early-diverging angiosperm lineages (for example, fossil and living Hedyosmum and other members of the Chloranthaceae) - could hardly be more simple" (Friis and Crane, 2007).1 Magnoliopsida%% (basal dicots)
B Schmeissneriales (parallel-veined leaves; fossil)
2 Amborellales
3 Hydatellales (monocot-like leaves - one midvein?)
B Sanmigueliales (monocot-like leaves; fossil)
C Nymphaeales
4 Archaefructales (dicot-like leaves; fossil)
5 Trimeniales
B Austrobaileyales
C Illicales
6 Ceratophyllales (etc.)...
Origin of Bisexual Flowers
Most of the basal angiosperm families in the ANHITA group are either dioecious (unisexual flowers) or contain species that are dioecious: Amborella, Hydatellaceae, Austrobaileyaceae, Chloranthaceae, Ceratophyllum, Buxus, and other basal eudicots (Friis and Crane, 2007). Whether this simplicity reflects the basal phylogenetic condition or ecological adaptation is uncertain. Most Mesozoic gymnosperms were dioecious (unisexual cones and reproductive axes), although some (e.g. Bennettitales and Irania hermaphroditica - interpreted by Schweitzer, 1977, as a ginkgophyte and possible angiosperm ancestor) were clearly monoecious (bisexual). How the bisexual flower evolved in the Nymphaeales, for example, is a mystery unless one considers an unusual discovery in the Late Triassic of a plant that produced homologous unisexual cupules with either ovules on the inside or pollen organs on the outside: Archaestrobilus cupulanthus (Cornet, 1996).
Archaestrobilus cupulanthus (from Cornet, 1996): A. Female cupule showing a central ovule, ventral suture, and food boddies around the distal rim of the cupule; B. Male cupule, showing microsporophyll units attached to outside of cupule, no ovule inside cupule, and a ventral suture; C. Cross section of a male cupule showing hair-like structures within cupule, but no ovules; D. microsporophyll unit showing a pair of bracts enclosing a cluster of pollen sacs at the end of a filament; E. pollen sacs at end of a filament, containing simple monosulcate pollen.
We may be seeing a similar situation preserved in the Chloranthaceae if the carpel is homologous with the cupule in Archaestrobilus. In this extant family we have Sarcandra, an unusual genus where the stamen attaches directly to the side of the carpel (although its vascular supply is separate). A similar condition exists in Chloranthus, although the stamen (bract or filament) is sterile on female flowers and the carpel is sterile on male flowers, indicating an ancestral bisexual condition similar to Sarcandra. And we have unisexual flowers in Hedyosmum, where an axis is covered with stamens (as opposed to an axis covered with unistaminate flowers). If this central axis in Hedyosmum is homologized with an elongated sterile carpel (cf. male cupule: B-C above, for Archaestrobilus), the bisexual condition for angiosperms would become basic. In support of this interpretation, the reproductive structures of Sanmiguelia lewisii are similar in structure to those of Hedyosmum (see below).
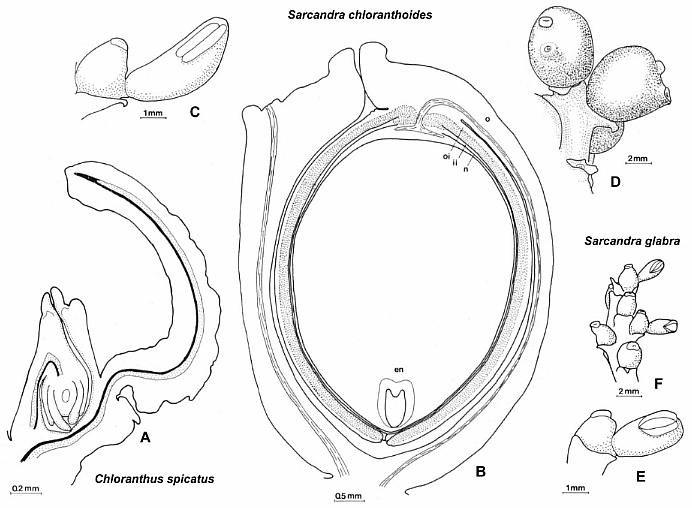
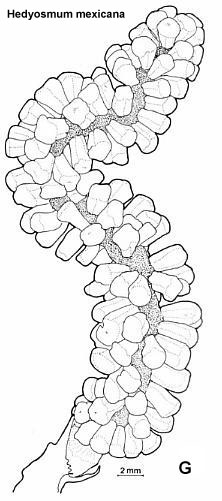
Illustrations of Chloranthus, Sarcandra, and Hedyosmum reproductive structures (Chloranthaceae), reproduced from Endress (1987) with permission.
Comparison of Synangispadixis tidwellii (male organs of Sanmiguelia lewisii; Late Triassic), Hedyosmum mexicana (extant), and Archaestrobilus cupulanthus (Late Triassic). Illustrations of Hedyosmum from Endress (1987) with permission. Illustrations of Synangispadixis tidwellii from Cornet (1986 and 1989b). Illustration of Archaestrobilus cupulanthus from Cornet (1996).
Basal Angiosperm Root Parasites?
Extant holoparasitic angiosperms are found in 19 angiosperm families in 11 orders, ranging from the Asterids as far down the angiosperm phylogenetic tree as the Piperales and Laurales, but not lower. Most have a pantropical distribution. The Monocots and the ANITA group (basalmost angiosperms) do not have any known extant holoparasites (Nickrent, 2005). The occurrence of parasites in so many divergent branches suggests a strong genetic tendency among core angiosperms for this type of habit and ecological niche. Therefore, it should not be surprising to find a previously unknown parasite related to the Monocots or to the ANITA group, or even a fossil root holoparasite related to extinct basal angiosperms. Gandolfo et al. (2002) describe a fossil achlorophyllous saprophyte: Mabelia connatifila (lived symbiotically with mycorrhizal fungi) from a Turonian coastal plain flora of New Jersey; they place it in the Triuridaceae (Alismitales).
Above cladistic tree modified and revised from Nickrent (2005). Sanmiguelia lewisii is shown on the branch leading to Monocots. Controversy exists over the placement of the Winterales, which some botanists link to the Saururaceae and Asaroideae (closer to the branch for the ITA), while molecular data link the Winterales to the Piperales and Magnoliales (Eklund et al., 2004).
There is fossil evidence for just such a possibility - root parasites that existed in a Late Triassic paleotropical flora dominated by cycadeoids, cycads, tree ferns, and sphenophytes (Cornet, 1986; Cornet and Olsen, 1993; Cornet, 2003). Two enigmatic fossil plants from Virginia went unnoticed for 30 years, because 1) the work of their discoverer (Bock, 1969) was not taken seriously by other paleobotanists, and 2) the reproductive structures were erroneously described as araucarian (Primaraucaria wielandii) or cycadalean (Triassiflorites grandiflora) gymnosperms, taxa which are unremarkable in the Mesozoic fossil record. Cornet (2003) compares these taxa with Langsdorffia hypogae, Lathrophytum peckoltii, and Lophophytum mirabile, all members of the Balanophoraceae (Santalales).
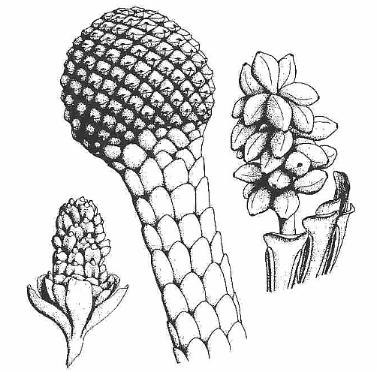
Above: Reconstructions of three types of reproductive structures grouped by Bock (1969) under Primaraucaria wielandii. Bock illustrates a specimen showing the inflorescence axis (center image: bract-shaped leaves bearing few functional stomata) attached to a tubor as in root holoparasites. Cornet (1986) recovered closed pedicellate carpels containing numerous seeds from the cone-like head. One reproductive structure even had appendages that resemble latrorse laminar stamens (above right).
Bock recovered his specimens of Primaraucaria and Triassiflorites from the same strata of the Richmond basin (Winterpock, VA) that produced diverse angiosperm-like pollen, which Cornet (1989a) describes under the Crinopolles Group. The association of these reproductive taxa raises the possibility that some of these unusual plants may be related to basal angiosperms.
Root holoparasites such as the pantropical Balanophoraceae are rejected by most botanists as having anything to do with the habits of the earliest angiosperms, and yet they may provide a partial answer to Darwin's "abominable mystery". Most root holoparasites have lost photosynthetic ability, and have greatly reduced numbers of stomata on achlorophyllous bract-shaped leaves. Their reproductive structures, however, can be quite elaborate or complex (Nickrent, 2005), sometimes highly reduced, and their pollen can be morphologically diverse, reflecting pollen morphology of their closest angiosperm relatives. The monocots are also characterized by loss of genes for a central root system and reduction of leaves to leaf sheaths or bracts, something that is characteristic of holoparasites. Thus, the monocots may have undergone some of the genetic changes (losses) that can lead to holoparasitism, and this reduction occurred very early in angiosperm phylogeny.
Lathrophytum peckoltii
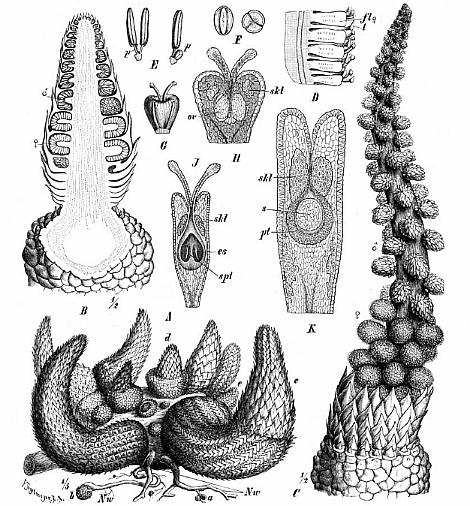
Lophophytum mirabile
Above: Two genera of Balanophoraceae, showing a strange mixture of paedomorphic and derived characteristics.
Endosperm Development
The term "endosperm" designates the tissue formed during the development of an angiosperm seed, which provides the essential nutrients utilized in embryo growth. An analogous food reserve is present in the seeds of gymnosperms. Even though it is referred to as "endosperm" in many botany texts, it is not homologous with the endosperm of angiosperms. In angiosperms endosperm is formed through double fertilization, where a second male gamete fuses with two or more secondary meiotic nuclei, forming a "triploid" condition. The fact that considerable variation exists among dicots and monocots in the number and position of polar nuclei in the embryo sac, and in how many secondary nuclei are involved in the fusion process to form the triploid condition, raises questions about when and how the triploid condition evolved.
Not only is there a wide variation between families (and in some cases genera: e.g. Plumago and Peperomia) in the number of polar cells generated during the formation of an egg cell, but the number of polar nuclei that join with the second male gamete is also variable (see figure below). Typically a triploid biparental endosperm develops, but recently a more primitive diploid biparental endosperm has been recognized (see quote from Williams and Friedman below).
Modified from Fig. 20-23 in Gifford and Foster, 1989: 595.
Furthermore, extensive comparative studies have revealed considerable variation in the mode of development of endosperm in angiosperms. Three main ontogenetic types are recognized (Gifford and Foster, 1989: 595-596):
Nuclar type: Free nuclear division without wall formation. In some plants several hundred nuclei are produced.
Celluar type: Division of the primary endosperm nucleus is followed by the formation of either a longitudinal or transversely oriented cell wall in the central cell, resulting in a row of cells.
Helobial type: Distributed throughout the monocotyledons (not found in dicotyledons). A basic type plus five variants are recognized. The primary endosperm nucleus is always found at the chalazal end of the embryo sac. The primary endosperm nucleus produces a transverse wall when it divides, which divides the cell into a small chalazal cell and a much larger micropylar cell.
Williams and Friedman (2002) recently demonstrated that not all angiosperms produce a triploid endosperm.
"In flowering plants, the developmental and genetic basis for the establishment of an embryo-nourishing tissue differs from all other lineages of seed plants. Among extant nonflowering seed plants (conifers, cycads, Ginkgo, Gnetales), a maternally derived haploid tissue (female gametophyte) is responsible for the acquisition of nutrients from the maternal diploid plant, and the ultimate provisioning of the embryo. In flowering plants, a second fertilization event, contemporaneous with the fusion of sperm and egg to yield a zygote, initiates a genetically biparental and typically triploid embryo-nourishing tissue called endosperm. For over a century, triploid biparental endosperm has been viewed as the ancestral condition in extant flowering plants. Here we report diploid biparental endosperm in Nuphar polysepalum, a basal angiosperm. We show that diploid endosperms are common among early angiosperm lineages and may represent the ancestral condition among flowering plants. If diploid endosperm is plesiomorphic, the triploid endosperms of the vast majority of flowering plants must have evolved from a diploid condition through the developmental modification of the unique fertilization process that initiates endosperm."
The variability in embryo sac development and endosperm formation implies parallel evolution rather than a linear evolutionary sequence of embryo sac types, since basal angiosperms produce diploid endosperm. The significance of triploid endosperm is not clearly understood, but its evolution may be related to the pollinator-induced radiation in the Cretaceous, when diversification occurred across family lines (implied in the composite cladogram above - Fig. 7).
Double fertilization has recently been documented in the Gnetales, which were once thought to be the Sister group of angiosperms. However, endosperm development does not occur from this second fertilization event.
"The coupled processes of double fertilization and postfertilization endosperm formation have long been viewed as important and synapomorphic features of flowering plants. Recent developmental studies of fertilization in the nonflowering seed plants Ephedra and Gnetum clearly document a regular process of double fertilization. The condition for Welwitschia remains unknown. Unlike angiosperms, the product of the second fertilization event in Ephedra and Gnetum is diploid and expresses the developmental program of an embryo. Explicit criteria for the evaluation of evolutionary homology indicate that the processes of double fertilization in Gnetales and angiosperms are homologous, having first evolved in a common ancestor of these two lineages." (Friedman and Carmichael, 1996)
Rather than providing evidence for a taxonomic relationship, the presence of double fertilization in a gymnosperm lineage that approaches the angiosperm condition in several organs (e.g. leaves of Gnetum), indicates that double fertilization may have been the ancestral condition within stem-group angiophytes, with diploid biparental endosperm evolving along several independent lines in crown-group angiosperms (Friedman and Williams, 2004).
Dicot-like Leaf Venation
In 1993 Cornet published a paper on a spectacular late Carnian dicot-like leaf fragment and possible flowers from the paleotropical Dan River - Danville basin of North Carolina and Virginia. In 2001 Brian Axsmith reported in an abstract the discovery of a relatively complete specimen of Pannaulika triassica from the Solite quarry from where the leaf fragment illustrated below came. He says the new specimen indicates an affinity with Clathropteris, which is a dipteridaceous fern. By comparing it to Clathropteris, he implies that P. triassica is a compound leaf, as Cornet predicted, but unless the new specimen is fertile (contains sori), its relationship to Clathropteris and ferns will depend on more than a superficial resemblance. For now Axsmith's interpretation needs to be considered until further study is published. Any superficial resemblance to pteridophyte leaves could be a holdover from a pteridosperm ancestry (i.e., Paleozoic and Mesozoic seed plants which bore fern-like foliage). Because the ancestors of angiosperms are not known, the older an angiosperm gets, the more likely it will possess or retain some ancestral features. Otherwise, angiosperm origin will become an enigma compounded if there can never be an angiosperm more primitive than any extant or Cretaceous angiosperm. Creating a new subclass, the Archaemagnoliidae, is a step in the right direction so long as its definition is not too narrow or its members are limited by age, i.e., the definition excludes fossils that appear too advanced or evolved in some organs for pre-Cretaceous angiosperms.
Pannaulika triassica from
the Late Triassic:
Why The Conflict Between Geneticists and Paleobotanists?
Recent molecular evidence indicates that angiosperms are not related to the Gnetales, destroying the Anthophyte theory that developed into dogma during the last two decades (Chaw et al., 1997; Hansen et al., 1999; Winter et al., 1999; Sanderson and Doyle, 2001; Bowe et al., in press). The Anthophyte theory had been tested and falsified, but not by paleobotanists. The falsification had to come from outside sources. The Anthophyte theory went like this: Angiosperms and gnetophytes had a common ancestry, making them Sister groups (Donoghue and Doyle, 2000). Doyle and Donoghue (1986; 1987; 1993) used the Sister group argument to support a Late Triassic (or older) origin of angiosperms, because as Sister taxa, angiosperms and gnetophytes had to have originated at the same time (the oldest recognized gnetophytes are Late Triassic in age; see Figure 15 above). Recognizing pre-Cretaceous angiosperms was still a problem for many paleobotanists who believed in a Cretaceous origin. Now that the Gnetales have been shown to be more closely related to conifers and not related to angiosperms at all, the believers seemed to have been given a reprieve. But not quite: That same molecular evidence also indicates that angiosperms have been separate from all other gymnosperms going back to (at least) the very origin of gymnosperms in the Devonian 400 million years ago (Sanderson and Doyle, 2001). As recently as 1997 some college biology textbooks show this independent origin for angiosperms, but still follow traditional thinking by indicating that angiosperms first arose in the Cretaceous (Cecie Starr, 1997).
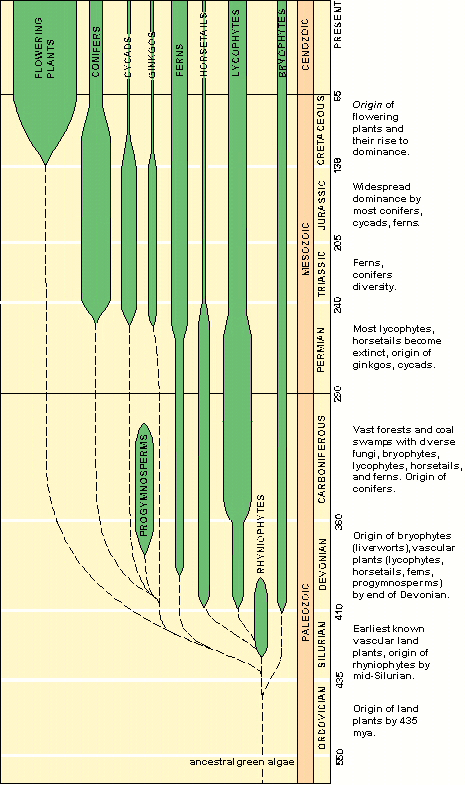
Modified from Cecie Starr (1997: Fig. 19.3)
Earlier genetic work on mutation rate had implied as much (an independent origin: Martin et al., 1989; Wolfe et al., 1989), raising the possibility back then that the Cretaceous origin theory was incorrect. And yet it took nearly a decade before paleobotanists began re-evaluating their concepts: "In conclusion, this study indicates that morphology may be more consistent with molecular data than it previously seemed." (Doyle and Endress, 2000; Doyle, 2001). If angiosperms are monophyletic back to Devonian progymnosperms or pre-ferns, then they and their direct precursors (however they will be classified when recognized) predate the Cretaceous by as much as 300 million years (Martin et al., 1989; Martin et al., 1993; Sanderson and Doyle, 2001). The Late Triassic angiosperm-like fossils Cornet described are 100 million years older than the age of angiosperm origin (i.e., 125 mybp) proposed in the Cretaceous theory, and fit nicely into this extended chronology. In fact, Wolfe et al. (1989) place the age of angiosperm origin at 230 mybp, two million years older than when the Crinopolles Group evolved based on lake cycle chronostratigraphy!
So, why is there still so much resistance (e.g. Sun et al., 1999; 2002) to the recognition of pre-Cretaceous fossils that could belong to the angiosperms or to their non-gymnospermous precursors if angiosperms did not evolve from any known group of gymnosperms (i.e., Gnetales, Bennettitales, Cycadales, Pteridospermales, and Coniferales)? Has the Cretaceous origin of angiosperms become a myth perpetuated by fear of uncertainty? Why can't paleobotanists be more honest and say that they don't have the answer yet to angiosperm origins, rather than bias the subject by saying "early" angiosperms and "primary" radiation in the Cretaceous - or worse by shifting the age of possible Jurassic strata to conform to a Cretaceous origin? If angiosperms evolved in the Triassic, Early Cretaceous angiosperms would be middle age. And if the genetic roots of angiospermy extend all the way back to the Silurian (see diagram above), that would make Cretaceous angiosperms "late bloomers".
Popular Theories Die Hard
There may be another reason besides scientific considerations for the belief in a Cretaceous origin. Funding by the National Science Foundation (NSF) in the United States requires that the research be feasible. That is, there must be a reasonable chance for success at achieving objectives. Sometimes a bare bones pilot study is done by principle investigators in order to show NSF officials that positive results can be obtained, but that more money is needed in order to flesh out a theory. NSF rarely funds a research project that is based on too much speculation, no matter how good the foundation for the theory may be. One cannot expect a project to be funded if an investigator says he wants to look for a type of fossil in a new area or in older age rocks. Usually what is required are some fragmentary fossils to show that with more searching additional and better material should be found.
In other words, those looking for angiosperm fossils in the Cretaceous know that they will be found there, because angiosperms existed during that Period. Those same paleobotanists would not go looking for angiosperms in Jurassic or Triassic age rocks if they think that angiosperms did not exist before the Cretaceous. And so the environment at NSF, which is made up of scientists who evaluate research proposals, is necessarily biased by belief carried over from the dominant opinion among experts, i.e., the reviewers of grant proposals. It's not unlike the man walking his dog at night, and coming across a neighbor down on his hands and knees looking through the grass in his front lawn. The man with the dog asks his neighbor what he is looking for, and he replies, "my car keys." Wanting to help, he gets down and begins looking with the neighbor. After awhile with no success, he asks his neighbor where he last had his keys, thinking that he could retrace his steps. The neighbor says, "over there," pointing to a different part of his lawn. The man with the dog then asks, "Why are you looking here for your keys?" The neighbor replies, "because I am closer to my porch light, and I can see the ground better where there is light." In this parable, keys = oldest fossils, and light = funding.
Funding is paramount for survival in the academic world of scientific research. It is paramount in any type of scientific research. Without money, a scientist cannot do research, and without a job or income, the scientist cannot do professional science. Convincing an NSF program director that a grant proposal to look for the origin of angiosperms is feasible, requires that scientists look for new evidence where the light still illuminates the ground. And for now that ground is called Cretaceous. More than likely a proposal to look for Jurassic angiosperms will be turned down by NSF, if not because of feasibility (80% of grant proposals are rejected), then because of bias among those scientists selected by NSF program directors to review proposals.
A search for Jurassic and older angiosperms will not be funded until there is a consensus among paleobotanists that the oldest angiosperms will be found in Jurassic or older rocks, and not in the Cretaceous. So far, the discoveries Cornet made of angiosperm-like pollen in the Late Triassic and Late Jurassic are not good enough to change herd mentality. Additional discoveries will be needed, such as the recent disclosure of angiosperm-like pollen in Middle Triassic strata of the Barents Sea (Norway: Hochuli and Feist-Burkhardt, 2004), and the discovery of a Nuphar-like leaf in a basal Jurassic lake bed in southwestern Utah. The discovery of well-preserved reproductive structures for the enigmatic Late Triassic plant, Sanmiguelia lewisii, is not enough if belief in a Cretaceous origin cannot be successfully challenged. Belief over science will prevail, despite the fact that fossils of Sanmiguelia show it has all the preservable characteristics of angiospermy. Dr. Peter Crane told Cornet back in 1993 at a paleobotanical conference in Iowa, "You will not convince anyone here that Sanmiguelia is an angiosperm." This is not the kind of statement the public expects from the former head of the Chicago Field Museum. The reason for that dogmatic statement is that there is no megafossil evidence from the Jurassic which proves angiosperms existed then (with the possible exception of Archaefructus). The gap in data is too great. The lily-like pollen and dicot-like (acanthoid) pollen Cornet found in some clay samples from the type section for the Upper Jurassic (Oxfordian) in France are not good enough, because they resemble too closely the pollen of more derived living angiosperms. Compare the images below.
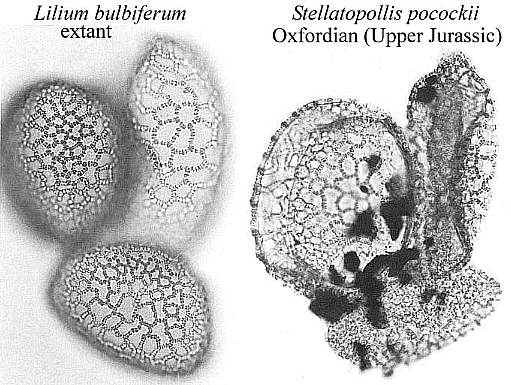
Contamination will be assumed by skeptics despite other evidence which disproves contamination, especially when no megafossil plant remains from similar beds can be mustered to back up the pollen.
Consequently, despite the discovery of angiosperm-like fossils in the Jurassic and Triassic, funding for searches in the Jurassic will not occur - until those at NSF are satisfied that the oldest angiosperms will not be found in the Cretaceous. That will require a paradigm shift and/or a major indisputable discovery in the earliest Cretaceous of fossil angiosperms too advanced to be the oldest angiosperms (if molecular chronology can be accepted by paleobotanists, and the Magnoliales and Winterales are recognized as existing by the late Neocomian, that milestone has already been passed). A paradigm shift will require the lawn nearest the porch light to be searched completely again and again until it is accepted that the key must lie beyond the search area. In other words, the Early Cretaceous does not contain a record of all basal angiosperms, despite claims to the contrary (cf. Crepet, 2000). Until that happens those who think angiosperms have a more extensive pre-Cretaceous record have to fund their research out of pocket and with little support from their peers.
For now, belief in the Cretaceous origin of angiosperms = funding, and funding means Darwinian survival.
The search for the oldest angiosperms and their ancestors is far from over, even though many experts in the field of paleobotany may still believe that 98% or more of angiosperms evolution occurred in the Cretaceous (i.e., back to Amborella). The discoveries made by Cornet in pre-Cretaceous rocks continue to be ignored by these paleobotanists and unchecked through additional collection and analysis. Geneticists have already falsified the Cretaceous Theory of Angiosperm Origin, making it only a matter of time before a paradigm shift occurs. An incremental shift in belief has begun with some botanists pushing angiosperm origin back to the Middle Jurassic (Soltis et al., 2005; De Bolt et al., 2005; J.A. Doyle, pers. Comm., 2005).
It has become clear through paleobotanical contributions such as Brenner (1996) and Sun et al. (1998; 2002) that the answer to Darwin's 'abominable mystery' will not be found in the Cretaceous. Extensive research by Lupia and others has given us the clearest picture yet of the Cretaceous angiosperm radiation, but that radiation is not dependent on a theory for angiosperm origin and early (basal) evolution. New knowledge regarding insect pollinators and their times of diversification is providing an alternative explanation for the radiation of angiosperms in the Cretaceous (Grimaldi, 1999; Crepet, 2000; Labandeira et al., 2001). Problem: The bias and dogma that has developed in the paleobotanical community has been more a hindrance than a help in solving this mystery, with an almost religious-like attitude evolving about the non-existence of pre-Cretaceous angiosperms (i.e., an attribute of dogmatism).
Problematic assumptions and faulty logic have contributed to this problem, requiring more objectivity and inclusiveness in the future. The assumption that tectate-columellate-reticulate exine structure first evolved in monosulcate pollen is a prime example (e.g. Foster & Price, 1981; Ward et al., 1989). Moreover, the significance of pre-Cretaceous fossils has not been based on the merits of the fossils, but on "how such fossils fit into [preconceived] phylogenetic trees" (Doyle and Donoghue, 1993). If the evidence does not support one of those trees, it has been either discounted as "not clear enough" or dismissed as "uncertain." Who would have guessed that polyplicate pollen could be basic to angiosperm pollen evolution (do a survey), or that the Middle and Late Triassic could yield so much evidence for angiosperm pollen evolution, or that a water lily could exist in the basal Jurassic? Not anyone who restricted their search (and beliefs) to the Cretaceous.
Angiosperm taxonomy is going through a major revision in evolutionary hierarchy based on the falsification of old schemes by molecular data. Phylogenetic trees have changed considerably in just the last five years (e.g. Doyle and Endress, 2000; Doyle, 2001). The Magnoliales, for example, were once thought to contain basal angiosperms, while the Nymphaeales were thought to be more derived than the Magnoliales. As recently as a decade ago the Chloranthaceae, Schizandraceae, and Trimeniaceae were thought to contain basal-most angiosperms, but now hold a more derived status than the near-basal Nymphaeaceae. With the discovery of Archaefructus - an aquatic angiosperm - in possible latest Jurassic strata containing fossils of feathered dinosaurs, water-lily-like leaves (cf. Nelumbonaceae) in Barremian-Albian strata of Virginia (Hickey and Doyle, 1977) no longer seem out of place. And with these changes, controversial pre-Cretaceous fossils, such as diverse angiosperm-like pollen throughout the Late Triassic, pre-Eudicot flowers and inflorescences (Axelrodia and Tidwellii) in the Norian, and Nuphar-like leaves in the basal Jurassic (Hettangian), seem to fit much better than before. Is the nature of the problem any clearer now?
Again, the evidence presented thus far by those who believe in a Cretaceous origin indicates that their search will have to be extended into the Jurassic and older rocks (see Miller and the Permian Gigantopterids). The abundance of angiosperm fossils in the Cretaceous has spoiled many paleobotanists and palynologists, who must now focus on known but rare pre-Cretaceous fossils with angiosperm-like characteristics, or on making large collections from both new and old Jurassic, Triassic, and Permian localities, with an eye (and mind) open for plants which could be angiosperms or pre-angiosperms. Until that happens, Darwin's 'abominable mystery' will continue to rule.
Bruce Cornet currently lives near El Paso, TX, and teaches physical geology, historical geology, and evolution.
This page was created on 3 June 2002, and last updated on 05/05/2007.