
Go to Second Half
last updated on 11/24/2014
More
Publications (1973-2006)
When Did Angiosperms First Evolve?
by Bruce Cornet, Ph.D.
PREFACE
The article you are about to read contains many technical terms and concepts used by professional botanists and paleobotanists. A glossary is given at the end of this article.
Recent discoveries and analyses in molecular botany (genetics and biochemistry), plant morphology and systematics, paleoentomology, paleopalynology, and geology, together with all angiosperm-like fossils from the Mesozoic, have combined to falsify the Anthophyte Theory and Cretaceous Theory of angiosperm origin. New and old evidence are evaluated in order to show how they can be synthesized to paint a very different picture of angiosperm evolution than the one that has been taught in current college textbooks for more than two decades (or by your current online university). Similar to Alfred Wegener's Hypothesis of Continental Drift, which could not be accepted by the scientific community until a mechanism for plate movement was established, many botanists could not accept evidence for rare pre-Cretaceous angiosperms until a satisfactory alternative explanation to origin could explain the radiation of angiosperms in the Cretaceous. Insect evolution (e.g. Hymenoptera) is now the primary suspect for the driving force behind the radiation of angiosperms in the Cretaceous. Deciphering the early history of angiosperms also required the overturning of false concepts of angiosperm phylogeny by molecular biology. Paleogeographic and climatic reconstructions are used to show why the Jurassic was the 'Dark Ages' for innovative plant evolution above the family level, and why the invasion of Gondwana by basal angiosperms did not take place until the Neocomian.
Table of Contents
Introduction
A Mystery Compounded by
Assumptions
Angiosperm Pollen
Morphology and Wall Structure
The Oldest Cretaceous
Angiosperm Record
The
Thigmomorphogenic Hypothesis by John Miller
New Challenges and Opportunities for Innovative Taxonomy by John
Barrett
More Questions about a Cretaceous Origin
The Angiosperm Invasion of Gondwana
Paleoequatorial Climate During the Jurassic
Super Greenhouse in Early Jurassic
Basal Angiosperm Hierarchy
Molecular Evidence and Fossil Evidence Combined
Water Lilies? in the Basal Jurassic
The Triassic Angiosperm
Pollen Record
Pseudo-Tricolpate Pollen
Beetles - Incidental Pollination
Cheirolepid Conifer Adaptation
Competition with Cheirolepidaceous Conifers
Second half
Radical Changes in Pollen Morphology and Exine Structure -
implications
How Rapid Evolution Might Occur - a molecular perspective
The Threshold of Angiospermy
What Factors Caused the Delay in Angiosperm Radiation?
Evolutionary
Reversals and Archived Genes
The Significance of
Polyplicate Pollen
The Problem:
Recognition of Pre-Cretaceous Angiosperms
Implications of Pre-Cretaceous Fossils
Key angiosperm synapomorphies
Sanmiguelia lewisii
Angiospermous
characteristics
Multilacunar
node and growth habit
More Evidence for Late Triassic Angiosperms
A Mid-Jurassic
Angiosperm? with Monocot-like Leaves
Schmeissneria from early
Late Triassic? to Middle Jurassic
A Living 'Dicot' with
Monocot-like Leaves!
Origin
of Bisexual Flowers
Basal Angiosperm Root Parasites?
Endosperm Development
Dicot-like Leaf Venation
Why The Conflict
Between Geneticists and Paleobotanists?
Popular Theories
Die Hard
Conclusions
References
Glossary
Addendum: What About Lequeria elocata?

Influential paleobotanists: Henry N. Andrews (left) and Elso Barghoorn (right), 1979, Harvard Forest, MA.
 During the 1960s, Professor Elso Barghoorn at Harvard, his colleagues and palynology
students (e.g. A. Traverse, J. A. Doyle and J. W. Walker) proposed an hypothesis about the
origin of angiosperms (flowering plants) that was simple and direct: Angiosperms could not
have existed much before their first accepted appearance in the fossil record (Scott et
al., 1960). Doyle was the first to publish on Early Cretaceous angiosperm
pollen and its evolutionary significance:
During the 1960s, Professor Elso Barghoorn at Harvard, his colleagues and palynology
students (e.g. A. Traverse, J. A. Doyle and J. W. Walker) proposed an hypothesis about the
origin of angiosperms (flowering plants) that was simple and direct: Angiosperms could not
have existed much before their first accepted appearance in the fossil record (Scott et
al., 1960). Doyle was the first to publish on Early Cretaceous angiosperm
pollen and its evolutionary significance:
"One of the major problems in the study of the evolution of higher plants is the paucity of evidence from the fossil record on the origin and evolution of the angiosperms. Because of the relatively sudden appearance of angiosperms in the fossil record, the lack of recognized angiosperm precursors, and the lack of any striking peculiarities of the macroscopic remains of Lower Cretaceous angiosperms (mostly leaves), almost all conclusions on the origin of the group and the nature of its primitive members have been based on comparative studies of its living representatives [e.g. Takhtajan, 1959; Cronquist, 1968]." (Doyle, 1969: p. 1).
Left: Jim Doyle on AASP field trip to Paluxy River, Albian fossil plant and dinosaur footprint locality near Glen Rose, TX, 1979.
M E S O Z O I C |
65
97.5 |
Upper |
Senonian |
Maastrichtian |
| Campanian | ||||
| Santonian | ||||
| Coniacian | ||||
| Turonian | ||||
| Cenomanian | ||||
| 144 | Lower |
Albian | ||
| Aptian | ||||
| Barremian | ||||
Neocomian |
Hauterivian | |||
| Valanginian | ||||
| Berriasian | ||||
| 163 | Upper Jurassic |
Tithonian | ||
| Kimmeridgian | ||||
| Oxfordian | ||||
| 188 | Middle Jurassic |
Callovian | ||
| Bathonian | ||||
| Bajocian | ||||
| Aalenian | ||||
| 201 | Lower Jurassic |
Toarcian | ||
| Pliensbachian | ||||
| Sinemurian | ||||
| Hettangian | ||||
| 231 | Upper Triassic |
Rhaetian | ||
| Norian | ||||
| Carnian | ||||
The
Cretaceous origin and radiation hypothesis became very popular among angiosperm botanists,
who had struggled to understand when and how the most important plants living today
evolved. Darwin had called the origin of angiosperms an "abominable
mystery," because he thought they had appeared full blown in the Albian, implying an
obscure pre-Cretaceous origin (Axelrod, 1952; 1961). Axelrod (1970) suggests that
angiosperms gradually entered the lowland record possibly due to climatic shifts (see also
Raven and Axelrod, 1974). Based on the distribution of Amborella (the most
basal extant angiosperm which populates island mountain slopes of New Caledonia), that
suggestion is not so easily dismissed today considering the evidence presented below.
To Darwin, who first proposed the theory of Phyletic Gradualism ("descent with modification"), the concept of rapid evolution must have seemed antithetic, even antiheretical for its day. The fossil record available to Darwin, however, was no where near as extensive as it is today, explaining in part his misconception (and that of others) about the grade of evolution for Albian angiosperms (e.g. Hickey and Doyle, 1977). And a good understanding of angiosperm phylogeny had not yet emerged in the mid-1800's through taxonomic studies of living angiosperms.
Above left: Dan Axelrod along the coastline near San Francisco, CA, 1978.
Largely through the works of Brenner, Chaloner, Couper, Crane, Crepet, Dilcher, Donoghue, Doyle, Friis, Habib, Herendeen, Hickey, Hughes, Jardiné, Lidgard, Lupia, Muller, Pedersen, Penny, Retallack, Tiffney, Traverse, Upchurch, Walker, Wolfe, and others in the later half of the 20th Century, going backwards in time, angiosperm diversity was shown to decrease rapidly with age below the Upper Cretaceous (i.e., Turonian = T in figures below), until angiosperm fossils are hard to find in Aptian- and Barremian-age sediments (Doyle et al., 1977; Doyle, 1999).


Gil Brenner (left, AASP, 1984), Bruce Cornet, and Jim Walker (right, Harvard Forest, 1979).
Peter Crane (left) and Bill Chaloner (right, University of Massachusetts colloquium, 1989).
S. Jardiné (left) and R.A. Couper (right, AASP, 1979).
Bruce Tiffney (left, AIBS, 1987) and Dan Habib (right, AASP, 1979).
Above: Al Traverse and Betty Traverse (Penn. State Univ., 1977).
Above: The late George Fournier, Chief Palynologist at Gulf Research & Development Co., Houston, TX (1979). He supported and helped Bruce Cornet in his research on the Crinopolles Group and on Sanmiguelia lewisii while at Gulf Oil (now part of Chevron).
During the 1970s the Cretaceous origin and primary radiation hypothesis became elevated to the status of theory as the amount of evidence in its support seemed to be unfalsifiable. There was no question that the angiosperms radiated in the Cretaceous to become the dominant plants inhabiting Earth. The fossil record showed that to be an empirical fact.
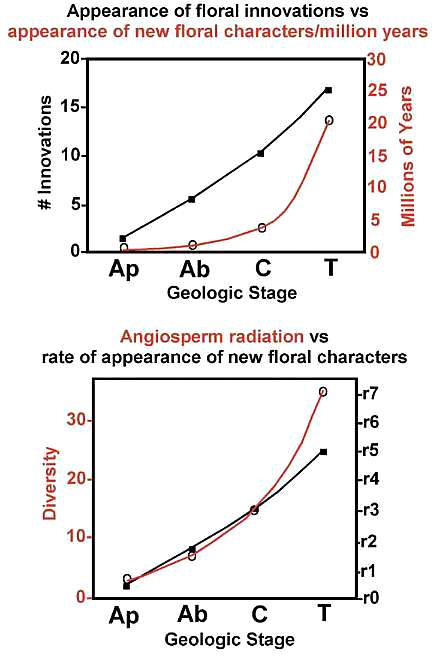
Above graphics modified (redrawn) from Crepet, 2000: Fig. 3. Ap = Aptian; Ab = Albian; C = Cenomanian; T = Turonian.
Lupia et al. (2000: 211) add further details: "Compilations of plant diversity, based on 197 macrofossil floras ranging in age from Late Jurassic to Paleocene, show a dramatic increase in the absolute number (summed diversity) of genera and species of angiosperm leaves through the mid Cretaceous (Fig. 15.2; Lidgard & Crane, 1988). The most pronounced increase occurred between the Barremian-Aptian and the Cenomanian. Within-flora percentages of angiosperms, based on the same macrofossil data, also increased rapidly through this interval, rising from near zero to about 70% (Lidgard & Crane, 1988 - see also Knoll, 1986). Trends in within-flora diversity, based on 860 Cretaceous palynofloras from middle paleolatitudes (25ş N - 65ş N palaeolatitude) in the Northern Hemisphere, show a broad similar pattern, but comparison of macrofloras and palynofloras from the same area shows that the increase in angiosperm species diversity is slower in palynofloras (Lidgard & Crane, 1990). In the Cenomanian angiosperms account for a mean of about 30% of the species in palynofloras, rising to levels of 40-50% or more by the late Campanian and Maastrichtian. In macrofloras the equivalent figures are about 60% and 80%. Similar results for palynofloras are also obtained based on an expanded data set of 982 samples from the Cretaceous of North America (Figs. 15.3, 15.4(a))."
From Hickey and Doyle (1977). Summary of Potomac Group leaf and pollen record (North America).
Acceptance or exceptance? That is the question. Unfortunately, exceptions to the theory were largely discounted or even ignored (swept under the rug even to this day), rather than investigated further in order to test and attempt to falsify the dominant theory (a requirement of the scientific method).
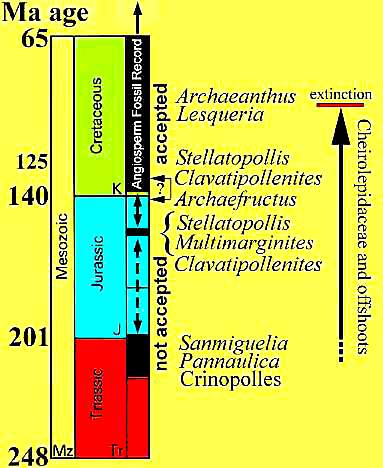
Just as not every geologist was convinced in the early 20th Century that Wegener's continental drift hypothesis was wrong, not every paleobotanist was sold on the idea that the cause behind the radiation of angiosperms in the Cretaceous was due solely to initial evolution.
"Flowers provide a record of mode of pollination in addition to revealing a precise knowledge of taxonomic affinity. In conjunction with a remarkably improved fossil record of insects (Grimaldi, D.A., 1999), the history of floral form provides a more precise knowledge of the timing of angiosperm-pollinator relationships and thus of angiosperm diversification vs. insect diversification... And this relationship, essentially unique to angiosperms, has been considered one of the foundations of relative angiosperm success... The pattern of angiosperm radiation is consistent with the pattern of anthophilous insect radiation and the pattern of appearance of derived floral characters and taxa specifically associated with the most advanced anthophilous insects. There is a compelling similarity between the rate of floral innovation/million years and the rate of angiosperm diversification during the Cenomanian/Turonian interval coinciding with the first occurrences of many derived insect pollinators." (Crepet, 2000). Bill Crepet at OSU in 1991.
Following the extinction of the Cheirolepidaceae in the Cenomanian, angiosperm diversity and floral innovation increased more rapidly. New fossil floral data reveal a dramatic modernization of the angiosperm communities by the Turonian (90 million years before present: Crepet, 2000). Examine a typical subtropical northern hemisphere Turonian coastal flora from eastern New Jersey at http://www.sunstar-solutions.com/sunstar/Sayerville/Kfacies.htm. Had Darwin witnessed such a pattern of diversification, he might have been even more astonished by the rapid ascension of flowering plants, which challenges the very mechanism he envisioned for evolution: descent with gradual modification. Was this spectacular event in evolution a product of initial origin and radiation, or were paleobotanists and palynologists too eager to find an acceptable explanation, even if that explanation ultimately proved to be wrong?
A Mystery Compounded by Assumptions
Upon closer examination of the evidence, however, the Cretaceous Origin and Primary Radiation Theory failed in its attempt to identify an angiosperm ancestor, and therefore the time of angiosperm origin. The theory (argument) was based on the assumption that scarcity of leaf fossils, the very-low angiosperm pollen-species diversity and abundance (percentage) in Neocomian-Aptian age strata (e.g. Brenner, 1996), and an absence of recognizable (accepted) angiosperm fossils in older rocks, equates to an absence of angiosperms in pre-Cretaceous strata.
"These observations have certain broad implications on the extent of pre-Barremian angiosperm diversification. On the one hand, some prior evolution was clearly necessary to produce the variation in size, shape, and exine sculpture seen in basal Potomac angiosperm pollen. In addition, as several authors have pointed out (Doyle, 1969; Muller, 1970; Walker and Skvarla, 1975; Doyle et al., 1975; Walker, 1976), there are extant magnoliid dicots with non-columellar monosulcate pollen distinguishable from that of gymnosperms only by TEM studies of endexine structure which may represent relicts of a still earlier, pre-columellar phase of angiosperm evolution not yet recognized (though not necessarily lacking!) in the fossil record." (Doyle, 1977, p. 513).
More recently Crepet (2000) noted: "Regrettably, there is no confirmation of the basal angiosperm taxon based on an unequivocal progression of discrete identifiable taxa early in angiosperm history. Instead, reliable evidence of early Cretaceous angiosperms suggests a rapid initial diversitication with "eudicots" immediately following magnoliids (Crane et al., 1995). Among earliest recognizable taxa are the families Chloranthaceae and Winteraceae (Crepet, 1998). These families (and others) are basal in several morphology-derived phylogenies. Nonetheless, more recent discoveries of Barremian (circa 130 million years before present) Nympheaceae and Amborella-like flowers by Friis et al. (2000) are consistent with the results reported in the Barkman et al. paper. Futher support for Barkman et al. comes in the form of recently discovered flowers very similar to those of the modern genus Nymphaea in Turonian deposits from New Jersey."
Consequently, angiosperms must have originated not long before the oldest accepted evidence in the Cretaceous (Occam's Razor - see definition in glossary). Despite its popularity, this argument was flawed for a number of reasons.
Confirmation bias is where "problem solvers favor initial hypotheses and ignore contradictory information that supports alternative hypotheses or solutions. Even when we find evidence that contradicts a solution we have chosen, we are apt to stick with our original hypothesis.
"Confirmation bias occurs for several reasons. For one thing, rethinking a problem that appears to be solved already takes extra cognitive effort, and so we are apt to stick with our first solution. For another, we give greater weight to subsequent information that supports our initial position than to information that is not supportive of it (Gilovich, Griffin, & Kahneman, 2002)." (Feldman, 2008).
Angiosperm Pollen Morphology and Wall Structure
Vascular plant pollen and spore walls contain one of the most stable and decay-resistant biopolymers known to man. That is why palynologists can extract pollen from rocks using some of the strongest acids know, without damaging the pollen. Spores and pollen (palynomorphs) get trapped in fine-grained rocks, usually when they settle out of a column of lake or ocean water into bottom muds, which later become compressed into rock as more layers of sediment accumulate above them.
Palynology is the study of spores and pollen produced by recent and ancient plants. Through this study an enormous body of literature has resulted over the last 60 years. That literature documents chronologic (temporal) changes in pollen and spore morphology through the last 420 million years, which reflect global changes in vegetative composition and distribution.
Angiosperm pollen is quite distinct from pollen produced by most gymnosperms, both extant and fossil. Most angiosperms produce pollen with a double-layered pollen wall (endexine below ectexine) separated by vertical posts or columellae (columellar type). In the more primitive angiosperms another type of pollen exists that resembles granular types of gymnosperm pollen wall structure, but the structure of the innermost wall layer (endexine) is different. Doyle (1978) summarizes the primary differences between angiosperm and gymnosperm pollen wall structure in the diagram below.
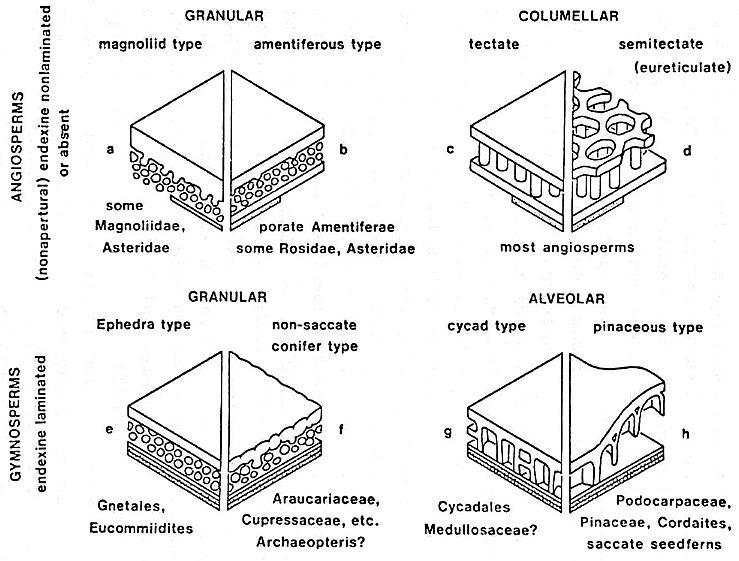
Because angiosperm pollen wall structure can be so distinctive and unique, it is the main reason palynologists were able to trace the radiation of angiosperms in the Early Cretaceous back to its roots. Moreover, these same pollen features have allowed the recognition of possible angiosperm pollen in pre-Cretaceous rocks.
The Oldest Cretaceous Angiosperm Record
Brenner (1996) shows that the oldest accepted angiosperm pollen (Liliacidites Group; Spinatus Group; Clavatipollenites Group; pre-Afropollis Group) that range from the Late Valanginian to Barremian (virtually the entire Neocomian or earliest Cretaceous) have all the typical exine characteristics of angiosperm pollen. Yet, in order to prove the Cretaceous origin theory, an angiosperm ancestor has to be identified. Instead of finding evidence for the ancestor - a gymnosperm predecessor with intermediate characteristics - the fossil record went largely silent, except for some enigmatic and controversial fossils further back in time that seemed like detached annoyances to those who wanted an orderly succession of "missing links" in the Cretaceous. The problem was that the list of real suspects for an ancestor was as long as the list of indisputable pre-Cretaceous angiosperms, until Doyle and Donoghue (1986) proposed their Anthophyte hypothesis of angiosperm origin, which targeted the gymnospermous Gnetales as the closest living relatives of angiosperms. The answer to Darwin's abominable mystery finally seemed to be within paleobotany's grasp, and by the mid 1990s paleobotanists considered the Anthophyte theory to be virtually proven (Doyle, pers. comm. 2002).
No sooner had that milestone in botany been reached than molecular biologists published data in the late 1990s which falsified the Anthophyte theory (e.g. Winter et al., 1999)! In addition, molecular biologists sent botanists back to the drawing board regarding angiosperm ancestry: "None of the extant gymnosperms is a direct ancestor of the angiosperms. Diversification of the modern gymnosperm orders occurred after they split from angiosperms." (Chaw et al., 2000). That same molecular evidence also indicates that angiosperms have been separate from all extant gymnosperms going back to (at least) the very origin of gymnosperms in the early Carboniferous 330 million years ago. That means that the ancestors of angiosperms cannot be called gymnosperms without direct fossil evidence for a gymnospermous ancestor. The neutral term, "angiophyte", is proposed, because it neither implies a gymnospermous ancestry nor a crown-group grade of angiosperm evolution.
| Pre-1999 | On the Sidelines | Post-1999 | Fallout |
| Most botanists and paleobotanists think that pre-Cretaceous angiosperms do not exist. The oldest Cretaceous angiosperms are thought to be most closely related to the Magnoliales and Chloranthaceae. Conclusion: The roots of most extant angiosperms did not evolve until the Cretaceous and Tertiary. | The Anthophyte Theory of angiosperm origin gains support. Angiosperms are regarded as the sister group to the ancestors of the Gnetales, and therefore had to exist by the Late Triassic. Despite such rationalization, pre-Cretaceous evidence for angiosperms is still debated: Crinopolles angiosperm-like pollen from the Late Triassic peaks interest, but without evidence for parent plants, botanists are unconvinced that they represent basal angiosperms. Descriptions of reproductive characteristics for Sanmiguelia (see below) are also doubted, because to accept them at face value would mean that crown group angiosperms had to exist by the Late Triassic - a controversial idea. | According to new molecular data, the least derived (basal- most) extant angiosperms are members of the ANITA group (Amborella, Nymphaeales, Illicium, Trimeniaceae, and Austrobaileya). The Ceratophyllaceae and monocots are considered less-derived than the Magnoliaceae and Winteraceae, while the Chloranthaceae and Eudicots (e.g. Nelumbo, Platanaceae, Proteaceae, and Papaverales) are given an intermediate position between the ANITA group and monocots. Flowers/fruits of monocots (Araceae), Nymphaeaceae, and possibly Amborellaceae are found in Early Cretaceous strata. | Molecular studies shake up the paleobotanical and botanical communities. The Anthophyte theory of angiosperm origin is falsified. The Cretaceous Theory of Angiosperm Origin is falsified. The new fossil discoveries in the Early Cretaceous give support to molecular-derived phylogenetic trees and data, indicating that most basal angiosperms evolved prior to the Cretaceous. Molecular clock data also indicate that important mutations common to many angiosperms first occurred in the Late Triassic and Early Jurassic. Estimated ages for specific angiosperm clades using molecular estimates are also generally older than inferences from the fossil record. |
Above: Olsen (vertebrate paleontologist), Ash, and Dilcher (paleobotanists) checking out enigmatic and controversial Triassic plant locality in June, 1980. Sanmiguelia was later dismissed based on alleged poor preservation, but its fossils are better preserved than most Cretaceous angiosperm fossils (Cornet, 1986; 1989). That false opinion was put forth by those who had much to lose if the Cretaceous Angiosperm Origin Theory was falsified.
 The recent discovery of Archaefructus
from an Upper Jurassic (144 mybp) freshwater lake deposit in China by Sun, Dilcher, Zheng,
and Zhou (Science Nov 27 1998) was a major find. This genus has
more recently been described under two names: Archaefructus liaoningensis and A.
sinensis, and is represented by complete plants from roots to fertile shoots (Sun et.
al., 2002). The reproductive axes of these plants lack petals and sepals, and
bear stamens in pairs below conduplicate carpels. Sun et al. (2002: 902)
hypothesize that Archaefructus may have been a submerged aquatic (like some
Nymphaeales) because of its dissected (Parsely-like) leaves and being found intact in a
lake deposit. They think that the lack of petals and sepals may be an ancestral
characteristic, but the highly specialized leaves, paired stamens, and habit of this plant
make that hypothesis unlikely.
The recent discovery of Archaefructus
from an Upper Jurassic (144 mybp) freshwater lake deposit in China by Sun, Dilcher, Zheng,
and Zhou (Science Nov 27 1998) was a major find. This genus has
more recently been described under two names: Archaefructus liaoningensis and A.
sinensis, and is represented by complete plants from roots to fertile shoots (Sun et.
al., 2002). The reproductive axes of these plants lack petals and sepals, and
bear stamens in pairs below conduplicate carpels. Sun et al. (2002: 902)
hypothesize that Archaefructus may have been a submerged aquatic (like some
Nymphaeales) because of its dissected (Parsely-like) leaves and being found intact in a
lake deposit. They think that the lack of petals and sepals may be an ancestral
characteristic, but the highly specialized leaves, paired stamens, and habit of this plant
make that hypothesis unlikely.
The genus was provisionally placed in the subclass Archaemagnoliidae, but later classified as a new basal angiosperm family, the Archaefructaceae. More recent age revision of the "Jianshangou Bed" shifted it to Early Cretaceous (Swisher et al., 1999; Sun et. al., 2002). In an attempt to make the data conform to theory, Sun et al. (submitted) revised the age for Archaefructus to range from 144 mybp to a youngest estimate of 125 mybp (124.6 mybp based on radiometric dating). 125 mybp is given by these authors as the most conservative estimate for the age of angiosperm origin. Interestingly, Sun et al. (1998) were uncertain that Archaefructus is an angiosperm (because of its age?): "Before it can be accepted unequivocally as an angiosperm, the nature of the winged fruits or seeds must be clearly understood, and we conclude at this time that it most probably is an extinct genus of Gnetales." "Most probably"? Or is this an example of laying the groundwork for plausible deniability if the "Jianshangou Bed" really is Jurassic in age?
Here is what Doyle and Endress (2007, Abstract) say about Archaefructus:
Integrating Early Cretaceous fossils into the phylogeny of Recent angiosperms
Doyle, James A. and Endress, Peter K.Asteropollis pollen are linked with Hedyosmum (Chloranthaceae), Anacostia is nested in Austrobaileyales, and Archaeanthus is linked with Magnoliaceae. Among Albian eudicots, Sapindopsis, Nelumbites, and Spanomera appear related to Platanus, Nelumbo, and Buxaceae, respectively. Affinities of dispersed pollen types are less robust, but our data support relationships of Walkeripollis tetrads with Winteraceae and Liliacidites (monosulcates with finer sculpture at the ends of the grain) with monocots. However, other fossils seem less closely related to previously suggested relatives. Virginianthus may be sister to either Calycanthaceae or the remaining Laurales; either result implies that reticulate rather than psilate monosulcate pollen was ancestral for Laurales and eumagnoliids as a whole. Mauldinia may be sister to both Lauraceae and Hernandiaceae rather than Lauraceae alone. Couperites (with “Clavatipollenites” pollen) may be related to Chloranthaceae, but positions in the basal ANITA grade are equally parsimonious. Plants with coarsely reticulate Pennipollis pollen appear related to Chloranthaceae rather than monocots, while Appomattoxia (with pollen resembling Chloranthaceae but with a continuous tectum) is more parsimoniously placed near Chloranthaceae or Amborella than in Piperales. Our analysis does not address the possibility that Archaefructus is basal to all living angiosperms, but its most parsimonious position within angiosperms is with Hydatellaceae, recently linked with Nymphaeales, indicating an early trend toward floral reduction in an aquatic habitat.
I have no problem with the theory that angiosperms underwent their first major radiation and diversification in the Cretaceous to which all extant flowering plants owe their existence. Could it be that there was a mass extinction (bottleneck in angiosperm evolution) at the Triassic-Jurassic boundary that preceded a major radiation (Fowell et al., 1994)? High levels of atmospheric CO2 have been detected at this boundary. Bottlenecks place considerable stress on surviving members of a group of organisms, limiting their diversity and causing the surviving members of that group to adapt to new climatic and environmental conditions. Barrett and Willis (2001) state that higher levels of CO2 might increase the evolutionary and selective pressures on plants owing to the induction of (abiotic) stress from which plants cannot escape. Evolving to cope with the changing environment might have been the only option for plants (De Bodt et al., 2005). High rates of conserved mutations in angiosperms for molecular 18S positions immediately following that mass extinction in the Early-Middle Jurassic could be interpreted as just such an adaptive response (see below).
It used to be assumed by botanists that the Cretaceous radiation was due to angiosperm superiority over all other seed plants (i.e., gymnosperms) once the first angiosperms evolved (a vestige of Darwinian thinking). However, the concensus opinion is shifting towards symbiotic relationships with insects in order to explain the rapid increase in angiosperm abundance and diversity in the mid-Cretaceous, which involves similar evolutionary advances across familial lines. That means evolution was not linear and sequential, but that separate evolutionary lines were being similarly affected by an outside agent. It was also difficult for botanists belonging to the Cretaceous School to reconcile a minor status of pre-Cretaceous angiosperms in world floras (i.e., less than 2%) with their abundance in the mid to Late Cretaceous. If only basal angiosperms and gymnosperms survived a future extinction event (minus most grasses and derived monocots), modern floras would once again become dominated by gymnosperms, and angiosperms probably would be no more abundant than they were in the Early Cretaceous (i.e., Albian).
One major characteristic of the angiosperm radiation is that there are few if any detectable extinctions of whole lineages (two types of pollen, belonging to the genus Proteacidites and to the Triprojectate complex, do show substantial species losses in the latest Cretaceous, but neither group goes extinct across the entire North American continent: Lupia, 1999; Lupia et al., 1999). And as far as I am aware, most Cretaceous angiosperm fossils compare to some group, family, order, or class of extant angiosperms (with the exception of Lesqueria elocata, which may not be an angiosperm: see Addendum), which is one of the reasons why a Cretaceous origin has been so attractive (parsimony or Occam's Razor). Normally an evolutionary radiation involves experimentation with frequent extinction, but the Cretaceous angiosperm radiation is unique in having so many successful phylogenetic branches (one reason why Darwin called it an abominable mystery). By comparison, there are many extinct types of mammals found in the Paleocene, Eocene, and Oligocene during the initial mammalian radiation of the Tertiary. See also the Crinopolles succession diagram below, which contains examples of both gradual and puctuated evolution with many taxa becoming extinct.
Crepet (2000) and Grimaldi (1999) indicate (imply) that angiosperm success is not necessarily due to seeds being protected inside an ovary (gymnosperms such as the Cheirolepidaceae, Araucariaceae, and Ephedraceae effectively evolved an analogous condition), but rather due to a symbiotic relationship with insects, where feedback through insect-driven selection determined floral evolution, which accelerated pollinator evolution through Busselator-like dynamics. The missing ingredient in the Cretaceous radiation was intelligent selection, even though that intelligence may have been of the insect variety. That ingredient (dedicated species-specific flower pollinators) may have been weak or nonexistent prior to the Oxfordian (Late Jurassic). See diagrams below and Cornet and Habib (1992). Selection by humans has resulted in rapid diversification and subspeciation/variation in horticultural plants, dogs, cats, bovines, horses, etc., which to an outside observer might be mistaken for rapid evolution (i.e., radiation). Such increases in apparent diversity are precursors to speciation.
See John Miller's web page on how insects may be responsible for the initial evolution of angiosperms back in the Permo-Triassic: The Thigmomorphogenic Hypothesis.
The four main groups of insects involved in angiosperm pollination (in decreasing order of importance) are the Hymenoptera (bees), Lepidoptera (butterflies and moths), Diptera (flies), and Coleoptera (beetles). Note (in figures below) the presence of the earliest members of these groups in the Triassic (pinkish red), and how each group increases diversification in the Carnian (climatic optimum: early Late Triassic), Early Jurassic (post-mass extinction: Hettangian-Sinemurian), and the Late Jurassic (floral recovery: Oxfordian-Kimmeridgian) - before the common appearance of angiosperm fossils in the Aptian-Albian (Early Cretaceous). Does insect diversification track angiosperm evolution and radiation better than the accepted Cretaceous angiosperm fossil record? What do you think?
Above figures modified from Labandeira, Eble, and Santa Fe Institute, 2001.
NEW CHALLENGES and OPPORTUNTIES for Innovative TAXONOMY
by John Barrett (DCBR7@yahoo.com)
"By the time Willi Hennig passed away 1966, CLADISTICS was widely accepted as an important tool for evolutionary taxonomy, adding molecular techniques and computer methods to handle huge amounts of new information.
"Previously morphological taxonomists had sought to describe similarities and differences by the Linnaean system, including its prominent binomial Generic and Species names. It was sought to recognize relationships in higher categories, but the level of taxa depended on judgments of degree of similarity and difference that were largely PHENETIC.
"In the 1950s and 1960s most biologists probably without deep thought accepted broadly a Darwinian concept that evolutionary change is gradual, and typically people thought of point mutations of protein-encoding genes as a typical process.
"In the ensuing forty years biology has discovered many new processes that may offer challenges and opportunities for creative and innovative taxonomy. One dramatic development was the general acceptance of SYMBIOSIS explaining MITOCHONDRIA of more complex EUKARYOTIC organisms and also photosynthetic CHLOROPLASTS of ALGAE and HIGHER PLANTS and probably affecting the development of the NUCLEAR ENVELOPE and LARGER MULTICHROMOSOME NUCLEUS with HISTONES and other important regulatory features. Lynn Margulis played a major role here around 1970.
"Another change which may be a particular challenge for TAXONOMY was around the same time - ELDREDGE and GOULD called attention to the prevalence of highly PUNCTUATED evolution first in the FOSSIL RECORD, and then it clearly appeared in MOLECULAR DATA - one consequence is MOSAIC EVOLUTION or HETEROBATHMY, where a clade such as a plant family may have advanced wood characters, variable leaves, and conserved floral or seed features that evolve largely independently presumable because of selective factors. People wondered why more transitional forms like ARCHAEOPTERYX between dinosaurs and birds do not occur in the fossil record, and surely PUNCTUATED EVOLUTION is a major factor, that key changes take place quickly, and often in small geographically isolated populations. In this regard the work of Sewall Wright is significant as he studied small populations at times, whereas Ronald Fisher and J.B. S. Haldane and Drosophila researchers focused on mathematics of large populations.
"From the time of Linnaeus 1753 and his many precursors, taxonomy implicitly had room for punctuated evolution as the basis for the PHENETIC criteria that was described. But just when biologists and geologists were learning that PUNCTUATED EVOLUTION is very central to the usual scheme of nature, Computers and Molecular Biology and Mathematical Algorithms were turning bright young researchers to CLADISTICS. It can be argued that they were developing an Aristotelian, medieval, a-priori mathematical method that put a blind eye to direct observation of what is going on in the real world of OBSERVATION and EXPERIMENT. It was easier to sit at a computer than do morphological field work.
"For about two hundred years taxonomists have recognized the genera Victoria and Euryale in waterlilies Nympheales. It seems to me these names reflect real-world observation of a very substantial size difference and thorns and ecology of these taxa in comparison with their relations among the fifty-old species that have been placed in Genus Nymphaea. The age of this large genus is one reason I think it should be divided, even though the precise manner is not yet clear. I believe retention of the long-established generic names helps people understand the Phenetics and Punctuated Rapid Evolution of these groups, which impressed early workers. Here in the Pacific Northwest, we have many highways that are sharply curved and often slippery in wet weather. It is generally the custom to post signs to keep drivers alert and in proper lanes and traveling at reasonable appropriate speeds. State Highway Departments might save money by posting fewer signs, but accidents and injuries would likely increase. In a sense cladists are taking down useful road signs if they merge genera like Victoria and Euryale into large clades and dismantle the useful Linnaean binomials. I hear there are proposals for ways to make taxomony be faithful to cladistic branching while retaining some of the traditional names as useful signposts - so people wont get stuck on thorns of Euryale ferox and the two Victoria species. I would tend to retain names like Vinca, which was the source of the important cancer drug Vincristine, and perhaps avoid name mergers that have effects their original authors never intended - there were proposals to lump Bubbia, Belliolum, and Exospermum in Zygogynum - this is think was defeated on further cladistic study that these groups are quite old and morphologically different - but persons sensitive to word meanings might have objected that these other species do not have "yoked gynoecia". I think there was a proposal to link Convallaria with another genus whose name was inappropriate - perhaps scientists don't fundamentally care, but this violates the original concept.
"I have been thinking of a number of other major developments in biology since 1966 or 1970 that change the traditional Darwinian picture, though they may not impact taxonomy as much. Some examples - we now know that promoters and regulatory regions are very important in organismic evolution - things that control levels and timing of gene expression and locations in tissue types. We are learning about protein folding and chaperones that affect whether genetic changes will have major organismic effects. We are learning about RNA interference and epigenetics and chromosomal doublings and deletions and even whole genome duplications. Perhaps ages for classes, orders, families, genera need standardization, with intermediate grades employed more.
"Perhaps I will expand this another day. - John Barrett" (published with permission, February 2007; DCBR7@yahoo.com)
More Questions about a Cretaceous Origin
Some critics of pre-Cretaceous evidence for angiosperms ask why there are not more records of Jurassic angiosperms if they first evolved during the Triassic. If one examines floral diversity and the number of new families, orders, and classes of vascular plants in the Jurassic, and compares it to the numbers found in the Triassic and Cretaceous, it will be noticed that the Jurassic was a period of slow recovery after a mass extinction (more correctly termed worldwide plant community disruption) at the Triassic-Jurassic boundary (Fowell et al., 1994; Tanner et al., 2003). Recently (November, 2005) an international symposium on the Jurassic boundary events was held in Nanjing, China (see abstracts listed in references). Very little evolution above the family level occurred as Early Jurassic floras (survivors) in paleo-tropical and subtropical regions of the supercontinent Pangaea had to adapt to more arid climatic conditions.
Chart below shows increasing aridity in central Pangaea at the end of the Triassic, just before a mass extinction.
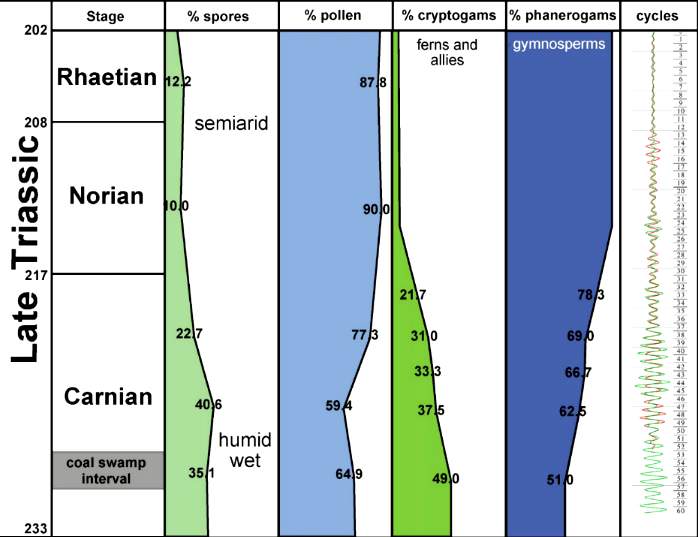
Fossil data taken from Cornet and Olsen (1990). Early to middle Carnian (Triassic) flora and fauna of the Richmond and Taylorsville basins, Virginia and Maryland, U.S.A. Numbered lacustrine-climatic cycles on right (404 K eccentricity cycles) from Olsen and Kent (2000) and Olsen (wall chart; pers. comm., 1999) for Newark basin, from Newark Basin Coring Project (1990-1993).
Early and Middle Jurassic floras of those regions are very similar at the genus and family level over large areas of the globe, being dominated by relatively few taxa, most of which were adapted for aridity with small leaves (microphyllous: Cornet and Waanders, 2006). The Australian (Gondwana) Jurassic palynomorph record (below) is typical.
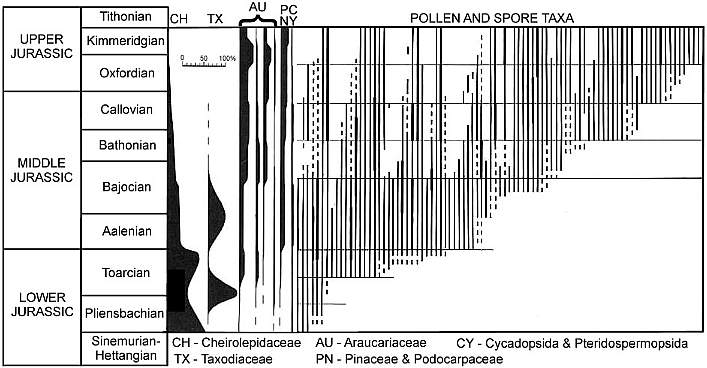
Above figure modified from Filatoff (1975: Text-fig. 5); Jurassic palynology of the Perth basin, western Australia. Plant diversity during the Hettangian-Toarcian was very low, with many Triassic survivors scattered around the globe. Gradually floral diversity increased with migration, repopulation, and evolution. See also Cornet and Waanders, 2006.
The Angiosperm Invasion of Gondwana
Gondwana is devoid of reports for angiosperm fossils prior to the Neocomian (Early Cretaceous). Only northeastern Gondwana (Isreal: Brenner, 1996) has palynological evidence for angiosperms there in the early Neocomian. Back in the 1980s and 1990s, it was thought that angiosperms first evolved in the tropics of Gondwana during the late Neocomian, and then migrated southwards and northwards, invading North America for the first time (Retallack and Dilcher, 1981; 1986). With better age and paleogeographic resolution, as well as data from the Triassic and Jurassic, it is now apparent that this scenario is incorrect. Rare basal angiosperms were widely distributed in Laurasia prior to the Cretaceous, but became restricted by climate change to wet tropical area around the Tethys Sea (northeastern Gondwana and southern Europe) during the Neocomian. A climatic barrier (expansive desert) in northern Gondwana prevented basal angiosperms from migrating further southwards before the late Neocomian. See migration figure below.
Hypothesis: It was not until the Late Jurassic when global climatic conditions improved that a symbiotic relationship between angiosperms and advanced insect pollinators (i.e., Hymenoptera) first evolved. A sudden increase in Hymenoptera diversity in the Kimmeridgian was followed by a sustained increase in their diversity through the Cretaceous. Refer to entomology figures above. It is that relationship which fueled rapid angiosperm and insect evolution and diversification in the late Neocomian, causing both to spill out of the tropics as desert barriers disappeared.
If one plots the distribution of Neocomian angiosperm megafossil and pollen localities on a Late Jurassic paleogeographic map (before Gondwana began to break apart), they straddle the semiarid, summer-wet equatorial belt: Above it in western Portugal (Friis et al., 1996; Friis et al., 2004), and northeastern China (Sun et al., 1998; 2002), and below it in Israel (Brenner, 1996), and in the Coco Beach sequence of the Gabon basin, west Africa (Doyle et al., 1977). Paleoclimatic reconstruction of the Gabon rift zone area during the Late Jurassic predicts high mountains with glaciers in the northern part of that region (similar to Mt. Kilimanjaro in northeastern Africa today), even though lowland areas (basins) were dominated by deserts, playa lakes, and savannas (Rees, 2001).
When one plots Middle-Late Jurassic localities that produce angiosperm pollen (e.g. Clavatipollenites, Stellatopollis, and Multimarginites: Vigran and Thusu, 1975; Cornet and Habib, 1992), they fall in the region of the North Atlantic rift zone between Canada and western Europe (especially northwestern France: western margin of the Tethys Sea). Early Jurassic angiosperm-like pollen from Sweden (Nilsson, 1958) and from the Hartford basin of Connecticut, USA (Cornet and Traverse, 1975) fall there and also to the south in the central Atlantic rift zone (Newark Supergroup).
Triassic angiosperm-like pollen and megafossils are distributed west-east across North America northwards to the Barents Sea (Cornet, 1986; 1989a; 1989b; 1993a; 1993b; Hochuli and Feist-Burkhardt, 2004). Fossil evidence for Sanmiguelia indicates that it is a basal crown-group angiosperm (Cornet, 1986; 1989b; see Sanmiguelia lewisii), implying that angiosperms first diversified in tropical areas of North America during the Late Triassic, but did not have dedicated pollinators that could accelerate their evolution as angiosperms had in the Cretaceous.
Recently, an angiosperm-like pollen grain (clavate-reticulate monosulcate) was discovered in a Carnian-age palynoflora from Namibia, South Africa (cores from the E. Tosha stratigraphic test well drilled by Superior Oil Company in the 1970s). It might indicate that some basal angiosperms managed to migrate to Gondwana prior to the development of migration barriers. Like Amborella today, they never became major elements of any flora.
However, no angiosperm-like pollen has been reported from Gondwana for the Jurassic. The most primitive living angiosperm, Amborella, from New Caledonia does not produce pollen that can be called angiosperm-like (Sampson, 1993), meaning that its ancestors and close relatives could go unrecognized in the fossil palynological record. The restriction of most Triassic angiosperm-like fossils to North America, the presence of angiosperm-like pollen in the Early and Middle Jurassic of western Europe, and the presence of distinctive (i.e., diagnostic) angiosperm pollen in western Europe during the early Late Jurassic (Cornet and Habib, 1992), imply that angiosperms first evolved in Laurasia. Some of them may have migrated northward with wet climatic zones as aridity spread across paleoequatorial and subtropical regions during the Jurassic. Coal swamps predominated at higher northern latitudes extending from Sweden to southern and northern China. Their floras were dominated by ferns, Bennettitales, and Cycadales (Wang, Y. et al., 2005; Wang and Li, 2005; Wan et al., 2005; Meng and Chen, 2005; Liu, 2005).
When wet tropics returned to northern Gondwana in the Early Cretaceous (beyond the circum-Tethys area), angiosperms could migrate south across the ancestral Straights of Gibraltar to northern Gondwana, island hop or move down the western shore of the Tethys Sea, and cross into the rift zone (Gabon basin) that was developing between South America and Africa. Timing was critical for the angiosperm invasion of Gondwana in the earliest Cretaceous: As climatic barriers to migration were disappearing, oceanic barriers were opening up due to rifting. Evidence for the first area angiosperms colonized in Gondwana indicates it was along the northeastern coast (Brenner, 1996), where a tropical maritime climate existed, based on laterite soil development.
Above figure is modified from Alister Rees (2001) to show distribution of paleoequatorial summer wet belt (speckled yellow-green) that was straddled by deserts in southern North America and southern Africa-South America, while a winter wet climate dominated southern Europe and the Middle East. Coal formation during the Jurassic was restricted to higher latitudes, starting with Early Jurassic coals in Sweden and Middle Jurassic coals in England (Yorkshire Delta), and extensive coal formation of Early and Middle Jurassic age in southern and northern China.
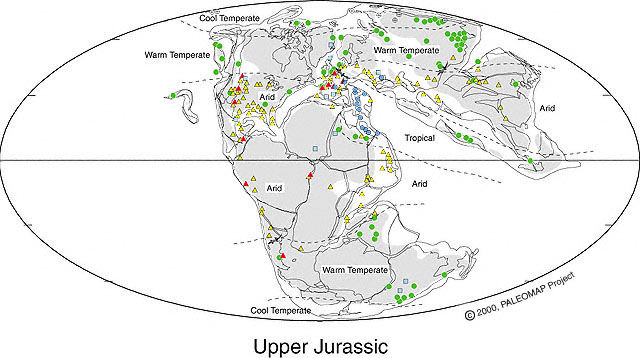
Above figure from PALEOMAP Project, with permission from Christopher Scotese (December 2005). This map shows laterite soil development (blue circles) along the northeast coast of Gondwana and southernmost Europe, indicating a wet tropical climate there at the time of angiosperm crossing. For legend see http://www.scotese.com/legend.htm.
Molnar (2001) considers south-east Asia as the center of origin for angiosperms in the Late Jurassic, but when Triassic and Early Jurassic evidence is considered, the center of origin is more nebulous (uncertain), and only a general Laurasian origin can be given. What he writes is nevertheless important in identifying possible strongholds for early angiosperms in the Late Jurassic:
"Although the precise location of the angiosperm center of origin is uncertain, it is predicted to be in south-central Asia (Takhtajan, 1969; 1987). Takhtajan (1969) has suggested that the 'cradle of the angiosperms' occurs somewhere between Assam and Fiji due to the high abundance of 'primitive' angiosperm families (e.g. Magnoliaceae and Winteraceae) found in that region of the Pacific basin. A serious flaw in the south-east Asian center of origin theory, acknowledged by Takhtajan himself (1969, p. 156), is the lack of fossil evidence. Hughes (1994, p. 22) has argued directly for the primacy of the fossil record to reveal events of the past. In contrast, determination of the center of origin, Takhtajan (1987) argues, must come primarily from distributions of extant taxa in a phylogenetic series, whereas the paleobotanical evidence can only reveal migration patterns (e.g. the equator-to-poles direction), but not the center from which the angiosperms initially spread. He considers south-east Asia (including Burma, Thailand, Indo-China, and Malaysia) to be the most likely region where angiosperms originated, since that is where the highest abundance of primitive angiosperms occur at present. Current distributions may not necessarily be similar to those of the past -- they may simply reflect 'centers of survival' rather than 'centers of origin' (Takhtajan, 1969, p. 158). Descendants of the first angiosperms may have dispersed far from their point of origin (Takhtajan, 1987)." Molnar (2001).

A. Takhtajan (left) and Arthur. C. Cronquist (right), University of Massachusetts, 1989.
 Although land bridges probably existed between the two supercontinents in the Late
Jurassic (Laurasia and Gondwana), it is hypothesized that a climatic barrier (i.e.,
aridity) in northern Gondwana prevented migration south (below the northeast coast of
Gondwana) prior to the Cretaceous. Only that coastline of Gondwana had a tropical
climate capable of supporting basal angiosperms, and it is there that the oldest
Cretaceous angiosperm pollen is found (Brenner, 1996). During the Late Jurassic and
Neocomian there was an interval of global cooling that could have aided the migration of
angiosperms. Evidence for this cooling comes in part from the withdrawal of the
Sundance Sea that crossed North America from the Middle to Late Jurassic. That
withdrawal may have been due to ocean water being locked up in expanding ice sheets at the
poles. The transgression of the Cretaceous Interior Seaway in the mid-Cretaceous
marks the melting or retreat of those ice sheets during a period of global warming.
Although land bridges probably existed between the two supercontinents in the Late
Jurassic (Laurasia and Gondwana), it is hypothesized that a climatic barrier (i.e.,
aridity) in northern Gondwana prevented migration south (below the northeast coast of
Gondwana) prior to the Cretaceous. Only that coastline of Gondwana had a tropical
climate capable of supporting basal angiosperms, and it is there that the oldest
Cretaceous angiosperm pollen is found (Brenner, 1996). During the Late Jurassic and
Neocomian there was an interval of global cooling that could have aided the migration of
angiosperms. Evidence for this cooling comes in part from the withdrawal of the
Sundance Sea that crossed North America from the Middle to Late Jurassic. That
withdrawal may have been due to ocean water being locked up in expanding ice sheets at the
poles. The transgression of the Cretaceous Interior Seaway in the mid-Cretaceous
marks the melting or retreat of those ice sheets during a period of global warming.
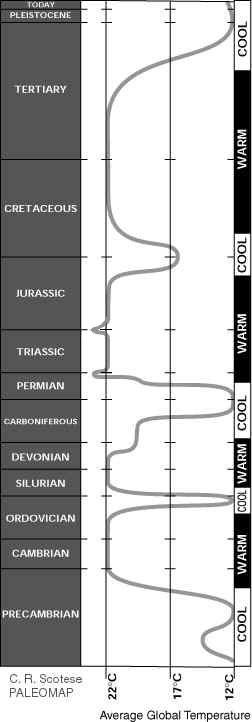
Left: Paleotemperature graph reproduced here with permission from C. Scotese (2005). http://www.scotese.com/climate.htm Temperature curve modified at Triassic-Jurassic boundary to match that at Permian-Triassic boundary - both times of increased carbon dioxide content of atmosphere and greenhouse effect.
Doyle (2000) shows the migration of the Winteraceae from the Early Cretaceous to the Tertiary. The oldest pollen assignable to the Winteraceae (Walkeripollis and Afropollis) is found in northern Gondwana (Israel, Saudi Arabia, and Gabon), while pre-Afropollis pollen is found in the basal Cretaceous of Israel (Brenner, 1996) along the northeastern coast of Gondwana. The Liliacidites Group and Clavatipollenites are also present in the basal Cretaceous (late Valanginian).
Because the Winteraceae are now placed above the Chloranthaceae (the probable source of Clavatipollenites) and above monocots, angiosperm evolution below the Winteraceae (i.e., Amborella, Nymphaeales, ITA, Chloranthaceae, Monocotyledonae, Piperales, and Magnoliales) had to have occurred before the Cretaceous, and prior to migration from Laurasia to Gondwana. Because the Liliacidites Group and Clavatipollenites are also present in the early Oxfordian (type section, basal Late Jurassic) of France (Cornet and Habib, 1992), evolution below the Piperales had to have occurred no later than the Middle Jurassic!
| Lower Cretaceous | Barremian | Presence of Winteraceae in northern Gondwana (Doyle, 2000); presence of Chloranthaceae and Araceae in southern Laurasia (Friis et al., 2004). |
| Hauterivian | Last chance for conventional migration to Gondwana as ocean barriers increase. | |
| Valanginian | Presence of pre-Afropollis Group, Liliacidites Group, and Clavatipollenites in northeastern Gondwana (Brenner, 1996). | |
| Berriasian | Climatic change allows migration from Laurasia to Gondwana. | |
| Upper Jurassic | Tithonian | Presence of Archaefructus in eastern China (Sun et al., 1998; 2002). |
| Kimmeridgian | Significant evolutionary rate increase for Hymenoptera (bees: Labandeira et al., 2001). | |
| Oxfordian | Presence of Liliacidites Group and Clavatipollenites in northern France (Cornet and Habib, 1992). | |
| Middle Jurassic | Callovian | Youngest age for angiosperm evolution below monocots. |
| Bathonian | Presence of Clavatipollenites in western Norway (Vigrans and Thusu, 1975). |
Molecular analyses of rbcL and 18S positions have been bootstrapped to determine when higher rates of angiosperm evolution or divergence occurred. It is based on concatenation of sequences from different genes. This approach has been shown to be extremely sensitive to addition or withdrawal of genes for a given number of species (Daubin and Gouy, 2001). Even though there are problems and limitations with this method, Sanderson and Doyle (2001) showed that there are three significant intervals of time for angiosperm innovation during the last 300 million years, based on an average rate of mutation for that period of time. The youngest (3rd position rbcL) is in the Cenomanian-Turonian at a time when Cretaceous angiosperm floras began to take on a more modern appearance (see Turonian Sayerville flora). The next oldest interval is Early-Middle Jurassic (18S), with a peak near the Early-Middle Jurassic boundary. Doyle (pers. comm., 2005) personally favors this interval for the time of angiosperm origin. The oldest interval, however, covers an overlapping period of time from Middle Triassic to Early Jurassic (2nd position rbcL), with a peak through much of the Late Triassic. It agrees with the first appearance of angiosperm-like pollen in the late Ladinian (latest Middle Triassic: Hochuli and Feist-Burkhardt, 2004), the rapid diversification of Crinopolles angiosperm-like pollen in the early Carnian (earliest Late Triassic: Cornet, 1989a), and the appearance of Sanmiguelia in the late Carnian - early Norian (Cornet, 1986; 1989a) - a plant that combines monocot vegetative and dicot reproductive characteristics. These data indicate that angiosperms or their stem group ancestors extend back to the Early Permian, and that most basal angiosperm evolution (below the Winterales) probably occurred in the Triassic and Jurassic.
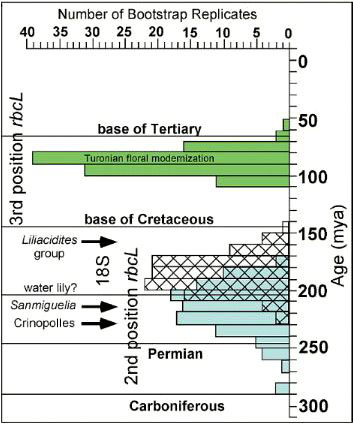
Above figure modified from Sanderson and Doyle (2001).
"Based on whole-genome analyses of Arabidopsis thaliana, there is compelling evidence that angiosperms underwent two whole-genome duplication events early during their evolutionary history. Recent studies have shown that these events were crucial for the creation of many important developmental and regulatory genes found in extant angiosperm genomes. Here, we argue that these ancient polyploidy events might have also had an important role in the origin and diversification of the angiosperms." (De Bodt et al., 2005).
Paleoequatorial Climate During the Jurassic
During the break up of Pangaea in the Mesozoic, tenuous and intermittent bridges for plant and animal migration existed between Gondwana and Laurasia. A consequence of plate movement and rifting was the creation of significant climatic and oceanic barriers to migration between these supercontinental fragments. As extension (crustal stretching) developed between North America, Africa, and South America in the Late Triassic, a Central Atlantic Magmatic Province (CAMP) formed in the basal Jurassic (Olsen and McHone, 2003). The underlying plate tectonic cause and Mantle heat convection may have had an influence on climate over this region (i.e., central Pangaea), especially in areas where horst-block mountains separated basins, creating rain shadow deserts over the basins as high mountains in the Basin and Range (rift) Province of southwestern North America do. Even though the heat budget from rifting would have been small compared to that of the atmosphere, increased dry air and heat within the rift zone (due to rain shadow effects plus heat flow from the Mantle) resulted in extensive evaporite formation beginning in the Middle Jurassic. The extremes in climatic indicators in the rift basins (i.e., eoleanites in the same formations as deep lake deposits), especially to the north in the Canadian Fundy and Moroccan basins, indicate environmental stress for plant communities there during the Early Jurassic. The Jurassic floras in those basins were all dominated by xerophytes (microphyllous conifers belonging to the Araucariaceae and Cheirolepidaceae).
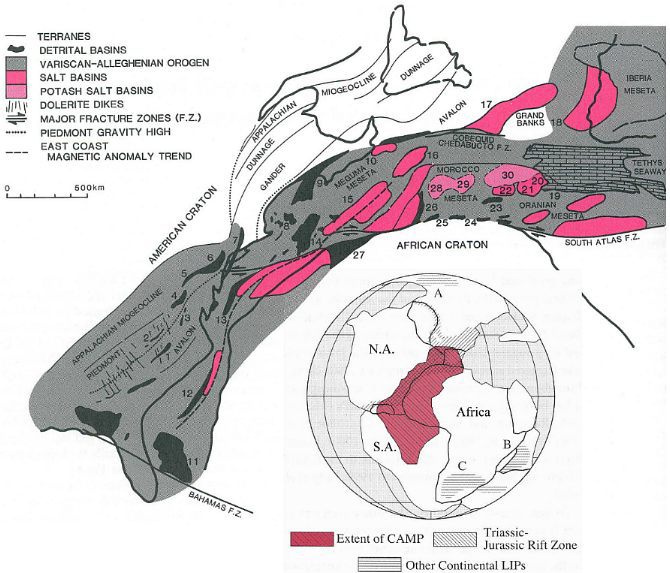
Above figures modified from Manspeizer and Cousminer (1988: top) and Olsen and McHone (2003: bottom).
With crustal extension and thinning over Mantle plumes of magma, heat flow at the surface increased all along the region where plate separation was occurring (and eventually oceans would develop).
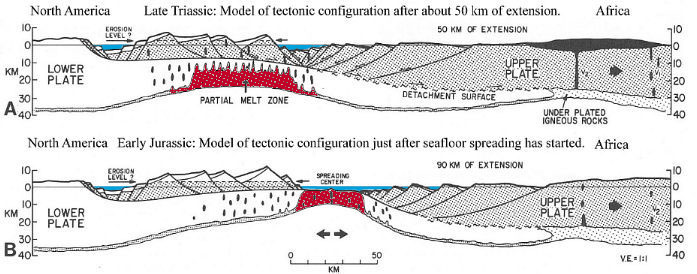
Above figure modified from Klitgord et al. (1988). A = extension and underlying magmatic development during the Late Triassic. B = extension after oceanic crust first developed during the Early Jurassic.
This heating occurred over a very large area, called the CAMP. In addition, with continental drift northwards for all parts of Pangaea, the paleoequatorial region gradually drifted into the arid subequatorial belt to the north, explaining in part the documented increase in aridity for central Pangaea during the Late Triassic and Early Jurassic (see chart above), and the loss of wet tropical areas along the former paleoequator (normally the region where an equatorial climatic low pressure area would exist, as it does today). See Rees (2001) and PALEOMAP Project. This climatic pattern would dominate much of northern Gondwana (except for northeast Gondwana next to the Tethys Sea) for most of the Jurassic.
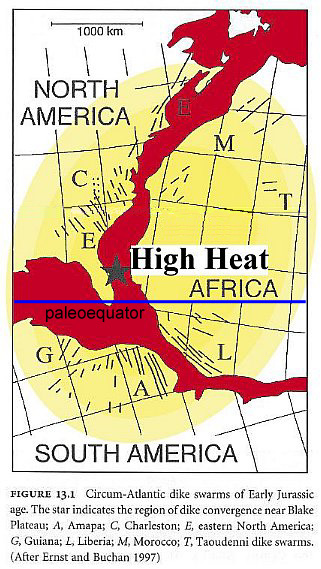
Above figure modified from de Boer et al., 2003.
Numerous evaporite deposits formed as sea water from the Panthalassa and Tethys seas (to the west and east, respectively) flowed into the rift zones of the proto-Atlantic and proto-Gulf of Mexico, where increased evaporation occurred. Thick deposits of salt formed in the proto-Gulf of Mexico during this period, which today are responsible for the hundreds of salt domes underlying the Gulf Coast and Gulf of Mexico. It wasn't until oceanic crust began to develop and significant amounts of moderating ocean water existed over the spreading centers during the Middle Jurassic that Mantle heat flow became less of a factor in affecting climatic. Climatic barriers to plant migration that once existed between southern Europe and northern Africa, and between North America and South America, gradually disappeared in the Late Jurassic and Early Cretaceous.
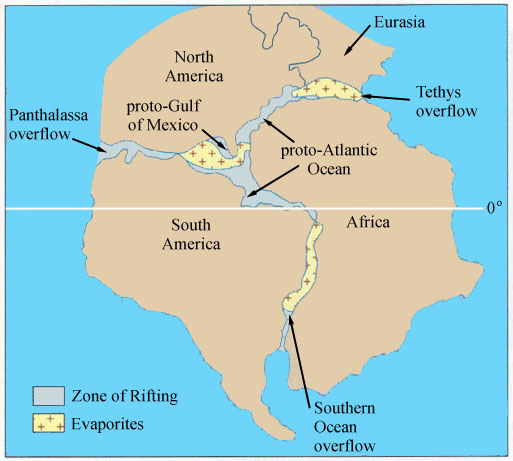
Above figure modified from Figure 23.3 of Monroe and Wicander (2001). Marine water from the Tethys Sea flowed into the central proto-Atlantic Ocean, while marine water from the Panthalassa (Pacific) Ocean flowed into the newly forming Gulf of Mexico and Caribbean. Marine waters from the south flowed into the proto-South Atlantic Ocean. The position of the paleoequator is shown near where it occurs today. During the Late Triassic the paleoequator was located much further northward in the middle of the Y-shaped rift zones (closer to the middle of the CAMP). As Pangaea gradually broke apart, the fragments all migrated northwards, and the tropics migrated southwards relative to the continents.
Super Greenhouse in Early Jurassic
Very little is known about paleoequatorial regions for the Late Triassic and Early Jurassic, because of the paucity of accessible deposits in that climatic belt. Deposits closest to the paleoequator (based on paleomagnetic inclination) are exposed mainly in the Santa Clara Formation of Sonora, Mexico (tropical Late Triassic tree fern-dominated paleoflora: Weber, 1985), the Richmond basin of Virginia (tropical Late Triassic tree fern-dominated paleoflora: Cornet and Olsen, 1993), the Saharan region of North Africa (subtropical paleoflora: Reyre, 1973), and in the Middle East (e.g. Saudi Arabia and eastern Egypt: tropical laterite soils). The South Georgia Triassic rift basin, N.A., was located at or near the paleoequator during its developmental history, but is entirely covered by younger post-rift formations. Its strata have been seen only in drill holes and seismic lines. The youngest strata encountered in that rift basin are mostly red beds. As Pangaea drifted northwards, subtropical and equatorial climates shifted southwards until northern Africa and northern South America were entirely within a broad arid belt by the Middle Jurassic - a climatic barrier to southward angiosperm migration. But what about climatic barriers in the Early Jurassic?
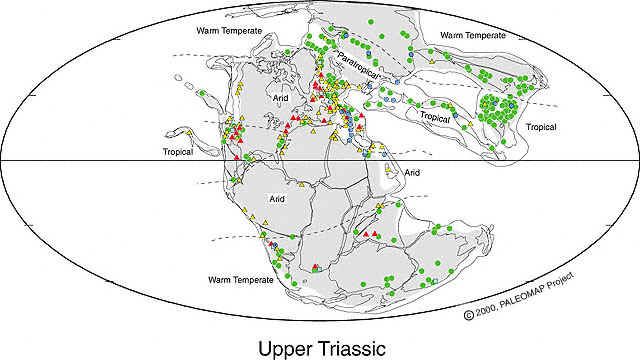
Above figure from PALEOMAP Project, with permission from Christopher Scotese (December 2005). Note the paucity of data points in northern Gondwana and at the center of rifting at the southern tip of Florida.
In addition to evidence for a mass extinction at the Triassic-Jurassic boundary (Cornet, 1977; Cornet and Olsen, 1985; Cornet, 1993; Fowell et al., 1994, Cornet and Waanders, 2006), including a fern spike, and Iridium anomaly - similar to what occur at the Cretaceous/Tertiary boundary, there is a large negative excursion in delta C13, indicating a major increase in inorganic carbon dioxide in the atmosphere (Whiteside et al., 2004; Whiteside et al., 2005). Global temperatures would have risen well above their present levels, and animals and plants living near or at the paleoequator would have experienced severe climatic stress (i.e very high temperatures and frequent flooding) that would have eliminated many species that survived the initial extinction event (Olsen, pers. comm., 2006). Those that did survive a significant global increase in CO2 would be forced to adapt or perish (Barrett and Willis, 2001). The paleoequator would have become a climatic barrier to plant migration between Laurasia and Gondwana. Work is currently in progress to determine the rate of atmospheric recovery and fall in global temperatures during the Early Jurassic. The source of the carbon dioxide is currently unknown, but the initiation of volcanism about 20 thousand years after the boundary (based on Milankovitch lake cycle stratigraphy) makes volcanic outgassing prior to the flood basalts (CAMP) the leading suspect.
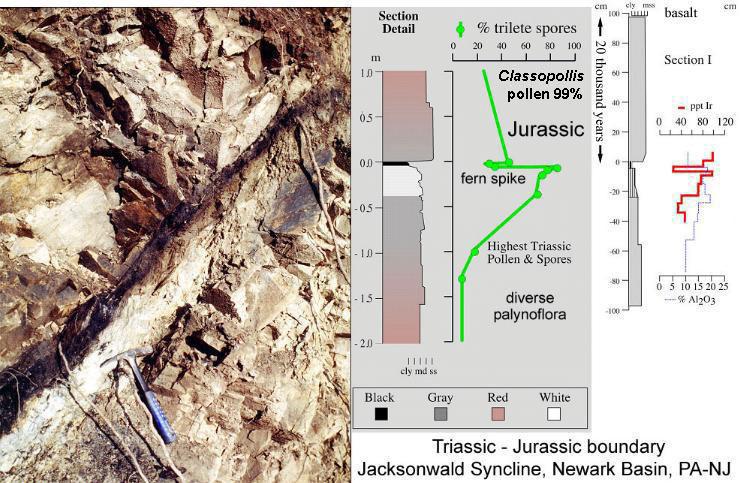
Above images courtesy of Paul Olsen and Jessica Whiteside, 2005.
Basal Angiosperm Hierarchy
Now that we have an accurate hierarchy for angiosperm evolution based on molecular biology (see below) and reliable fossil data from northern Gondwana for the Neocomian, we can determine the minimum level of angiosperm evolution at the time of angiosperm crossing to Gondwana. Previously, this timing was used as an argument that angiosperms did not exist prior to the Cretaceous, because they were not present in Gondwana during the Jurassic. But when angiosperms do first appear in northeastern Gondwana in the late Valanginian (Brenner, 1996), a significant amount (~80%) of basal angiosperm evolution had already occurred. The extent of that evolution (based on recent molecular data) is told by their living descendants, which include Amborella, Nymphaeales, ITA (i.e., ANITA group), Chloranthaceae, Eudicots, Monocots, Piperales, Magnoliales, and Winterales (Doyle and Endress, 2000).
As recently as a decade ago, the Magnoliales and Winterales had been considered more basal (primitive) than the Nymphaeales, Eudicots, and Monocots; and that interpretation supported an Early Cretaceous origin for angiosperms based on Cretaceous fossils (i.e., Magnoliaceae and Winteraceae in the Barremian-Cenomanian). Brenner et al. (2003) published an article "On the origin of angiosperms: The Euanthial and Paleoherb debate," in which they favor extant Magnolia as representing the link between flowering and nonflowering plants, even though molecular biology had falsified that possibility more than a decade earlier (Wolfe et al., 1989; Martin et al., 1993; Winter et al., 1999; Chaw et al., 2000; Sanderson and Doyle, 2001). Doyle (2005) presents a cladogram for the Euanthial theory, based on pollen evolution, which illustrates why it was so hard for those supporting the Cretaceous origin of angiosperms to recognize pre-Cretaceous angiosperms with Magnolia-like, Chloranthaceae-like, and Wintera-like pollen present in the Neocomian-Albian.
Modified from Figure 1 in Doyle (2005). This cladogram illustrates the now falsified theory of angiosperm origin based on the interpretation that the Magnoliales contained the most primitive living angiosperms. It illustrates why monocots, and pre-Cretaceous fossils bearing monocot-like characteristics (e.g. Sanmiguelia) could not be accepted as early angiosperms, because according to the above phylogeny, monocots are some of the most-derived angiosperms.
The Paleoherb hypothesis can explain the presence of a plant like Sanmiguelia in the Late Triassic, while the Euanthial hypothesis cannot. The presence of Magnolia-like flowers in the mid-Cretaceous can be explained through convergence (competition) with beetle-pollinated gynmosperms (see Beetles - Incidental Pollination below). See also Competition with Cheirolepidaceous Conifers.
"With four orders, 19 families, and approximately 8,500 species, the magnoliids comprise the largest clade of early diverging angiosperms... This eight-gene tree provides the first strong support for the monophyly of the four orders of magnoliids, and for sister group relationships between the Canellales/Piperales and Laurales/Magnoliales." Cai et al., 2006.
"Phylogenetic analyses using parsimony and likelihood methods and sequences of 61 protein-coding genes provided strong support for the monophyly of magnoliids and two strongly supported groups were identified, the Canellales/Piperales and the Laurales/Magnoliales. Strong support is reported for monocots and eudicots as sister clades with magnoliids diverging before the monocot-eudicot split. The trees also provided moderate or strong support for the position of Amborella as sister to a clade including all other angiosperms." Cai et al., 2006.
This concept that the Canellales/Piperales and Laurales/Magnoliales diverged before the monocot/eudicot split differs from that of Doyle and Endress (2000), which shows magnoliids diverging after the split. See cladogram below.
The desertification of the tropics in northern Gondwana would have prevented water-loving basal angiosperms from migrating to southern Gondwana during the Jurassic. Similarly, the distribution of wet tropical and subtropical habitats probably controlled the spread of the oldest angiosperms and/or their stem group ancestors across North America during the Late Triassic. Heat from rifting may have caused wet subtropical habitats to extend north along the Newark Supergroup rift zone to the Boreal realm, explaining the presence of angiosperm-like pollen there in the Middle Triassic (see Hochuli and Feist-Burkhardt, 2004). Any record of angiosperm-like pollen east of the Richmond basin of Virginia (Cornet, 1989a) would be deeply buried in the Continental Shelf of North America and Africa (i.e., inaccessible if not destroyed by burial metamorphism).
Above Figure 2 modified from Doyle (2000).
The distribution of angiosperms and gymnosperms between distant islands in the South Pacific indicates that short distances are not difficult to overcome, especially when sea birds carry seeds across those ocean barriers. Archaeopteryx first appeared in the Late Jurassic. It was not a powerful flier. Soaring probably carried it out to sea where it has been found preserved in back lagoon carbonate deposits. Pterosaurs did not appear until the latest Triassic, and are thought to have soared in the skies over open oceans. Nevertheless, sea barriers can be effective in limiting the types of plants to those whose tiny seeds can "jump" long distances on storm winds (e.g. cyclones), or be carried by fliers. The palms have overcome ocean barriers through the evolution of large seeds that can float, but that did not happen until late in angiosperm evolution.
Amborella trichopoda (the most primitive living angiosperm) exists only on the South Pacific island of New Caledonia. How did it get there, and when?
"More than 280 million years ago, at the end of long sedimentations and intense underwater volcanic activity, New Caledonia emerged near Gondwana, which would become Australia.
"New Caledonia was characterised by total isolation for more than 300 million years. This explains how nature there was able to preserve fossilised species. The territory has a wealth of original vegetation from some 2,500 endemic plants, and is therefore unique on the planet, making it the first « botanical garden » of the South Pacific, followed by New Zealand." Institut de Recherche pour le Développement / IRD
Could Amborella (or ancestors in the same family, as has been demonstrated for the Early Cretaceous: Friis et al., 2000) have crossed into Gondwana before heat from rifting helped create a climatic barrier formed by continental drift sometime in the Late Triassic (post-Carnian)? If this plant migrated to southern Gondwana in the Neocomian, why didn't other basal angiosperms closely related to Amborella (below the Nymphaeales) migrate also and survive on New Caledonia and other islands? More derived basal angiosperms (above the Nymphaeales) are present on New Caledonia and other South Pacific islands (e.g. Miller, 1989). "The big debate these days (concerning New Zealand too) is over how many of the "relict" groups are ancient vicariants dating back to continental fragmentation and how many got there more recently by long distance dispersal from Australia, etc." (J.Doyle, pers. Comm., 2005). Had more-derived basal angiosperms made that crossing back in the Triassic, one would expect that some of them might have evolved in parallel to angiosperms in Laurasia during the Jurassic and Early Cretaceous - but that did not happen, or there would be a fossil and extant record of novel basal angiosperms in Australia, New Zealand, New Guinea, Fiji archipelago, and New Caledonia similar to the diversification and dominance of marsupial mammals there. Thus, could Amborella help pinpoint the time of angiosperm origin in the Triassic?
Below: Postulated history of angiosperm evolution in the Mesozoic, showing the initiation of origin (Triassic) and the initiation of radiation (Cretaceous) related to the sequential breakup of Pangaea, the increase in aridity in the tropics due to Mantle heat flow and CO2 outgassing during rifting, and the loss of coal-producing environments (i.e., swamps and rain forests) in northern Gondwana due to northward continental drifting during the Jurassic, which acted as a climatic barrier to the southward migration of water-loving basal angiosperm such as Amborella and the water lilies.
Click on figure for enlargement.
Data in above graphic modified from Hickey & Doyle (1977); Doyle et al. (1977); Cornet and Olsen (1993); John C. Butler (1995); Alister Rees (2001); Hochuli and Feist-Burkhardt (2004).
Molecular Evidence and Fossil Evidence Combined
The current consensus opinion among botanists is that Amborella and the Nympheaceae (water lilies) are genetically the most primitive (basal) living angiosperms, but their morphological differences are so great that other variations and intermediates once must have existed.


Left: Nuphar xrubrodisca (photo courtesy of Barre Hellquist); right: Amborella trichopoda (photo courtesy of Porter Lowry).
If what is being discovered about angiosperms in the earliest Cretaceous is an indication of evolutionary modification of Jurassic survivors rather than de novo evolution from a pre-angiosperm, then the Triassic might contain types of angiosperms never before imagined, types which were rare and have no close relatives among living angiosperms (e.g. Sanmiguelia). That is because many of those taxa became extinct during the creation of the bottleneck (mass extinction at Triassic-Jurassic boundary: Fowell et al., 1994). As a group, those taxa more than filled the morphological gap between Amborella and the Nympheaceae.
This is what molecular biology is now telling us: There were at least eight (8) basal to early branches of the angiosperm phylogenetic tree (there were probably more that became extinct). Descendants of the survivors are represented today by (in evolutionary succession):
1) Amborella (least derived),
2) the Nymphaeales,
2b) the Hydatellaceae (new sister group to Nymphaeales),
3) the ITA (Illicium, Trimeniaceae, and Austrobaileya),
4) the Chloranthaceae,
5) the Eudicots (Buxaceae, Trochodendraceae, Nelumbo, Platanaceae, Proteaceae, etc.),
6) the Monocotyledonae + Piperales,
7) the Magnoliales + Winterales, and possibly
8) the Laurales (most derived).
Ken Kinman (pers.
comm. 2007) writes: "I was always a little nervous about evidence that Amborella
branched off alone at the base of crown angiosperms. That nervousness intensified
when it was found that it contains so many horizontally transferred genes. ANY
estimate of the age of any clade (containing Amborella as the only living group,
or Amborella and other living groups) is therefore to be taken with a grain of
salt. I won't really be very surprised if it split off after Nymphaeales, since I
had Nymphaeales at the base of Magnoliophyta back in the 1990's. And even if Amborella
is at the base of crown group Magnoliophyta, a younger age of Sanmiguelia is no
big problem given how long unknown ghost lineages can be. For all we know the Sanmiguelia
clade could
have split off from other angiosperms back in the Permian (although I really doubt its
ghost lineage goes back THAT far). Never know when a new fossil find might upset the
basal angiosperm "applecart"."
The deep and distinct roots of these eight had to have existed at the end of the Triassic (just before the mass extinction) if the cladistic tree of Doyle and Endress (2000: Fig. 7) is correct and if the Triassic fossils (Crinopolles Group, Sanmiguelia, Liliacidites, and Magnolia-like pollen) fit that evolutionary tree approximately where indicated in their composite diagram below (which has been modified to show the distribution of Sanmiguelia characteristics, a Late Triassic plant that combines dicot and monocot characters). Note: Their tree shows only the postulated relationships between extant angiosperms (as grades of evolution), and indicates nothing about the amount of branching (with extinctions) that may have occurred in the basal part of the tree. Furthermore, if Sanmiguelia is a crown-group angiosperm, many surprises await future Triassic fossil collectors. See "Recognition of Pre-Cretaceous Angiosperms" (part 2). These are testable hypotheses and/or predictions.
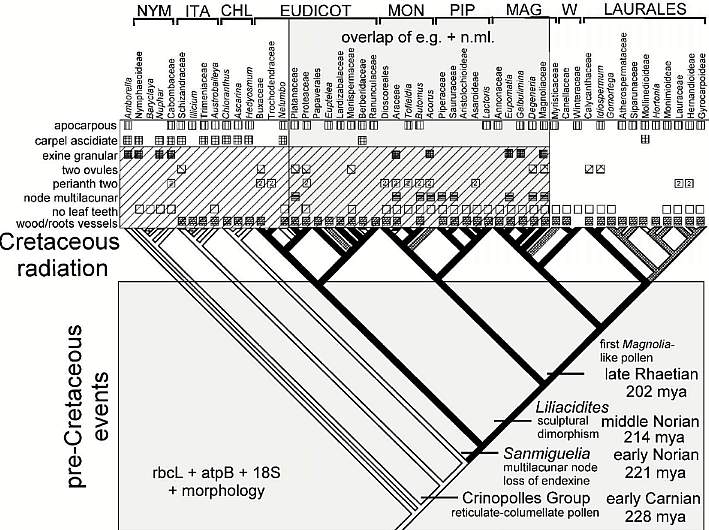
Modified after Fig. 7, Doyle and Endress (2000). NYM = Nymphaeales; ITA = named after Illicium, Trimeniaceae, and Austrobaileya; CHL = Chloranthaceae; MON = Monocotyledonae; PIP = Piperales; MAG = Magnoliales; W = Winterales.
Postulated angiosperm phylogenetic tree based on most recent taxonomic and molecular data (same branching as in above cladistic tree), put into chronologic context to show initial (basal) branching in the Triassic, no major branching in the Jurassic (genetic drift resulting from allopatric speciation appears to have occurred during the Early-Middle Jurassic; see 18S data above), and diversification across familial lines in the Early Cretaceous (due to symbiotic relationship with insect pollinators). Compare with insect diversification above. This diagram shows a minimum of eight species that survived the Triassic-Jurassic mass extinction, and which have living descendents.
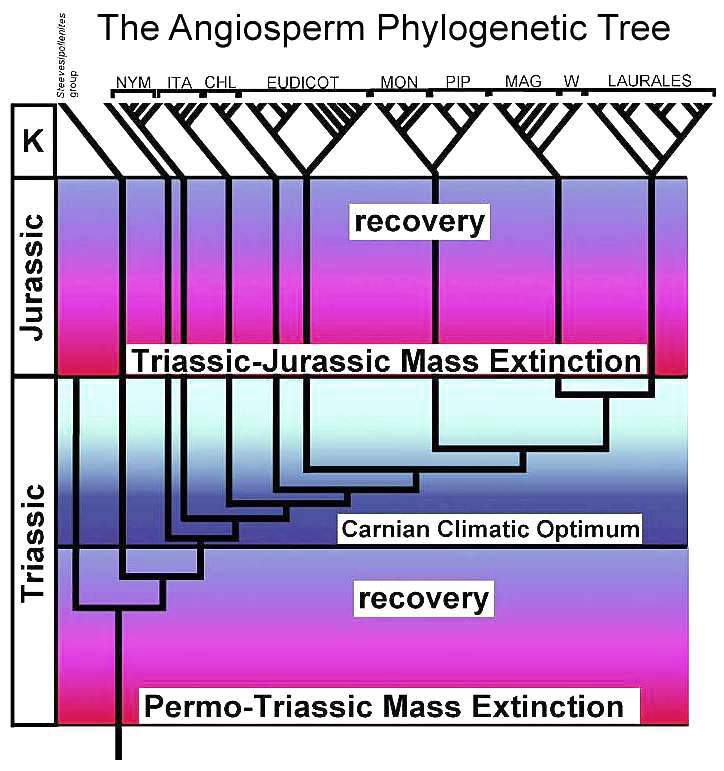
NYM = Nympaeales; ITA = named after Illicium, Trimeniaceae, and Austrobaileya; CHL = Chloranthaceae; MON = Monocotyledonae; PIP = Piperales; MAG = Magnoliales; W = Winterales.
On the one hand, some pre-Cretaceous angiosperms may appear to violate sequence events for the "first" appearance of organ characteristics in the Cretaceous (for example, leaf rank sequences deduced by Hickey and Doyle, 1977), but instead indicate that angiosperms could evolve and re-evolve those characteristics at different times (e.g. Pannaulika: Cornet, 1993).
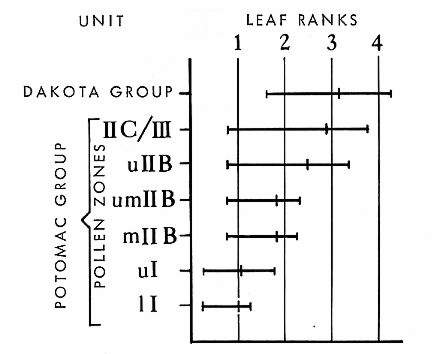
Left: Leo Hickey (during visit to Penn. State Univ. in 1976); right: from Hickey and Doyle (1977). Change in the range of leaf ranks shown by the Potomac and Dakota angiosperm floras.
 On the other hand, some pollen characteristics, which are thought to be derived within
extant angiosperms, might be very primitive. Sanchezia (Acanthaceae) and Spathiphyllum
(Araceae) produce specialized polyplicate pollen types (even with characteristic
auriculae) that have been traced back to the Triassic, and even to the Permian (Cornet, 1989a; Cornet and Habib, 1992).
For example, the tribe Trichanthereae (extant: Vasanthy and Pocock, 1986) produces
a very ancient pollen type: 80 um Sanchesia decora with auriculae
(extant below on left: 1, Vasanthy et al., 2004); Multimarginites sp. A
with auriculae (below circled) from the Late Jurassic (Oxfordian) type section (middle: 2,
Cornet and Habib, 1992); Cornetipollis cf. reticulata without auriculae
from the Carnian Richmond basin palynoflora (below on right: 3, Pocock and Vasanthy, 1988).
On the other hand, some pollen characteristics, which are thought to be derived within
extant angiosperms, might be very primitive. Sanchezia (Acanthaceae) and Spathiphyllum
(Araceae) produce specialized polyplicate pollen types (even with characteristic
auriculae) that have been traced back to the Triassic, and even to the Permian (Cornet, 1989a; Cornet and Habib, 1992).
For example, the tribe Trichanthereae (extant: Vasanthy and Pocock, 1986) produces
a very ancient pollen type: 80 um Sanchesia decora with auriculae
(extant below on left: 1, Vasanthy et al., 2004); Multimarginites sp. A
with auriculae (below circled) from the Late Jurassic (Oxfordian) type section (middle: 2,
Cornet and Habib, 1992); Cornetipollis cf. reticulata without auriculae
from the Carnian Richmond basin palynoflora (below on right: 3, Pocock and Vasanthy, 1988).
Above: Stanley A.J. Pocock (AASP, 1979).

This morphotype is too bizarre to have evolved independently in two separate and unrelated phylogenetic lineages (Vasanthy, Cornet, and Pocock, 2004). Hortonia floribunda (Monimiaceae) also produces pollen with a similar type of spiral-ribbed sculpture (Sampson, 1993). The simplest hypothesis which explains these data is that ancient characteristics were archived and preserved, later reappearing (as evolutionary reversals) when their genes become reactivated. Reversals become clues to phylogenetic ancestry.
A recent paper (Hochuli and Feist-Burkhardt, 2004) reports on eight pollen-grain taxa with angiosperm-like morphology (cf. Retisulcites) from marine Middle Triassic (early Ladinian) sediments of the Boreal Realm (Norwegian Arctic, Barents Sea area), and indicates that floral recovery from the Permo-Triassic mass extinction may have included the first angiosperms. Trisulcate?, operculate?, syncolpate, and monosulcate aperture types are present. One (pollen type A) resembles Polycolpopollis spp. from the Late Triassic (Carnian) Richmond basin (below),
From Cornet (1989).Figs. 89-93. Polycolpopollis magnificus Cornet 1989,89-91. Same grain (76X72 microns). 89. Low focus on two colpi joined to form a loop. 90. Median focus of nexinal body. 91. High focus on third colpus oriented at right angles to the others. 92. Holotype (81X 74 microns) High focus on two slub-parallel colpi that.approach one another at their ends; SGM-S1. 93. SEM (79X 73 microns) Portions of three colpi visible; large reticulum supported by columellae. SEM micrograph by J. W. WALKER.
Fig. 94. Unidentified fragment. (72 X 60
microns) A portion of a large, possibly polycolpate species,
perhaps related to Polycolpopollis. Note short broad columellae beneath a thick
reticulum.
while another (pollen type N) resembles Pentecrinopollis spp. from the Richmond basin (see below), but has a less-well-developed reticulum joining the heads of clavae. However, the Middle Triassic taxa occurred outside the tropics in a warm-temperate climate, which is unexpected.
Water Lilies? in the Basal Jurassic
In science hypotheses and theories must be predictive. That is, the cladistic-molecular phylogenetic tree above predicts that members of the Nymphaeaceae should be found in the Jurassic and even in the Carnian (early Late Triassic). Kirkland et al. (2002) report the discovery of a water-lily-like leaf in the basal Jurassic Whitmore Point Member of the Moenave Formation. It was discovered while excavating hundreds of dinosaur, reptile, and small mammal footprints preserved in red mudstone layers at the margin of a large but shallow lake bed. The locality is in St. George, Utah, and represents one of the best localities for Early Jurassic ichnofossils in the world.
Above: The dinosaur track site is located in the right center of the above image, directly above the truck (white tent area in the valley next to the main road).
Above: Location where the dinosaur trackway was found with one footprint containing the leaf fossil. Exfoliation from rain damaged the footprints and leaf fossil before they could be coated with preservative.
Above image: Eubrontes footprint with mud cracks. You are looking at the underside of the overlying massive fine sandstone unit that covered the tracks, producing casts of the positive prints on the underlying mudstone.
The leaf impression was found inside a dinosaur track impression. The fossil was photographed and casts made of it before it was destroyed. The picture below appeared in the Utah Geological Survey publication, Survey Notes (Kirkland, et al., 2002).



Above images and close-ups courtesy of James I. Kirkland, Utah Geological Survey.
This leaf impression resembles an extant Nuphar leaf (Nymphaeaceae), complete with petiole and impressions of radiating veins.

Above: Nuphar polysepala from Wyoming. Photo by Barre Hellquist.

Above: Nuphar polysepala from Wyoming. Photo by Barre Hellquist. Note footprint impressions in soft mud and how a lowering of lake level exposes the footprints and plants to drying and potential fossilization, if additional mud is deposited on top of the dried leaves and prints in a subsequent flood or rapid rise in lake level during a heavy rain.
The cast is shown below, along with the outline drawn of the dinosaur track in which the leaf impression was preserved. The leaf is 26 cm wide, with a petiole at least 18 cm long.

Above: A cast was made of the specimen before it was destroyed by rain. The outline of the dinosaur track is shown; it was within this track that the leaf impression was preserved. The leaf was either 1) washed into the track after it was made, or 2) it was sticking to the bottom of the dinosaur's foot until this step, where the leaf came off the foot and stayed with the imprint. The second explanation would explain the damage to the leaf lamina and squashed (splayed) appearance of the petiole. It would also mean that the dinosaur trekked through a patch of these water plants before coming out of the water onto the muddy bank of Lake Dixie.
The dinosaur trackway shows the direction from
which the theropod traveled before the leaf fell off the bottom of its foot. The
leaf was squashed and distorted (petiole splayed out) enough to indicate that it had
traveled with the dinosaur for a good number of steps (stuck on by mud and/or between the
dinosaur's toes). The relatively good condition of the fossil impression after
having been stepped on however many times between the "lily" patch and where the
detached leaf ended up speaks volumes on its durability (ruggedness and laminar
thickness). Nuphar leaves are equally durable (thick), making it an
excellent example for comparison.
Retracing the path of the theropod places it across E. Riverside Dr. in
a quarried area where dinosaur bones have been found, a sauropod trackway, many theropod
tracks, and what Andrew Milner pointed out as possible plant root casts. Those
"roots" resemble thick rhizomes (horizontal in situ stems) spreading
through the lake bed siltstone and mudstone layers. Although those rhizomes
are found 1.7 meters stratigraphically above the footprint horizon containing the
waterlily-like leaf (A. Milner, personal communication, 2006), the presence of possible
rhizomes in that area raises the possibility that a plant facies (niche) may have existed
there earlier. By excavating those layers, additional leaves (attached) and even
reproductive structures and seed pods might be found.

Above: A block of siltstone and overlying darker mudstone contains a cast of a rhizome (horizontal stem) bearing dozens of small rootlets. This specimen came from the rock layers below the sauropod trackway. The light gray aureole around the rhizome was caused by the reducing effect of decay, while the red cast represents infilled red clay after the rhizome decayed. At least one upwards branch might represent the base of a leaf petiole (the rest of the leaf may be hidden in this block or in its counterpart). Only through additional excavation and preparation of specimens such as this will it be determined if more of this plant is preserved in that unit.
If this fossil belongs to the Nymphaeaceae, it would not only confirm the basal position of the Nymphaeaceae based on molecular evidence, but also extend crown-group angiosperms back at least to the basal Jurassic. Additional specimens, along with reproductive structures are needed to prove the fossil is that of an angiosperm. Nevertheless, it establishes that this plant existed; if it was preserved once, it will probably be preserved elsewhere in these deposits. Need I remind the reader that Sanmiguelia was first known only from leaf impressions, and was erroneously diagnosed by the Cretaceous School because that type of preservation did not reveal key information, which came later from better-preserved specimens in Texas. The author has seen a similar type of leaf preserved in the Petrified Forest Member of the Chinle Formation (late Carnian or early Late Triassic).
The Triassic Angiosperm Pollen Record
Evolutionary radiations, small and large, fall into the category of Punctuated Equilibria. They involve rapid changes in genetic structure and organization which cannot be adequately explained by Darwin's Phyletic Gradualism ("descent with modification") model for evolution.
In the middle of Pangaea during the early Carnian (Late Triassic - 228 mybp) something extraordinary occurred. Within a paleo-equatorial (tropical) rift basin, distinctive angiosperm-like pollen suddenly appeared, along with numerous intermediate pollen types that briefly coexisted with a most unusual precursor pollen type: A polyplicate pollen type present today in the Araceae. Collectively they were placed by Cornet (1989a) in the Crinopolles Group (Crino = lily-like; polles = pollen). Angiosperm-like pollen, however, was rare, typically making up less than 2% of Triassic palynofloras, and in most cases less than 1%.
Consider the evidence in the illustration below: From auriculate polyplicate to reticulate-columellate monosulcate in about 100,000 generations, based on Milankovich-type climatic (lacustrine) cycles.
Click on image for a Crinopolles cladogram (for Mac users hold down left mouse key).
There are five reasons why the Crinopolles pollen data are important:
Genetic change responsible for evolution can only occur in gametes, because they are the cells which propagate the species. It is their genes that carry mutations and alterations in the genetic code. Anything that affects propagation will figure into species survival. Because pollen represents one half of the reproductive system, it couldn't be more front and central to mechanisms that favor increased diversity and evolution.
Formed by a coordination of sporophyte and gametophyte cellular processes, pollen wall structure, sculpture, and chemistry can play a critical role in reproductive strategies and their success. The pollen wall is usually the only part of the male gametophyte that gets preserved as a fossil (traces of cellular components have survived in some petrifactions). Because structure, morphology and function correlate in pollen, radical changes in exine morphology and structure must signal changes in either pollen delivery (i.e., mode of dispersal), place and mode of germination (e.g. spermatozoa vs. pollen tubes), or interaction with the female gametophyte and/or sporophyte. Depending on the types of exine change, corresponding changes in the female gametophyte can sometimes be inferred (but not proven).
Changes in pollen morphology and exine structure involve a relatively small group of interacting genes. Each morphological, structural, and sculptural characteristic of the pollen wall is probably controlled by one or more genes interacting in concert. Unlike other plant organs, much of which is rarely preserved or preserved intact, the pollen wall represents one of the best examples in the fossil record of microevolution: That is, evolution at the gene level (as compared to evolution at an organ system level), where the number of genes controlling morphology can be estimated and compared for each change.
The unusually complete and detailed stratigraphic record for the first appearance, evolution, selection, survival or extinction of 12 Crinopolles morphotypes over a geologic time interval of one Eccentricity cycle of the Equinox (~100,000 years: Olsen et al., 1996; Kent and Olsen, 2000), with the initial burst in Crinopolles diversity appearing over a time interval of perhaps only two Precession cycles of the Equinox (~50,000 years), is the first example ever documented of microevolution with time constraints in the fossil record (Cornet, 1989a; Cornet, 2000). Examples of both Phyletic Gradualism (step-wise evolution) and Punctuated Equilibria (bush-type evolution) are represented in the Crinopolles cladogram.
The Crinopolles Group may signal the time angiospermy first evolved about 230 mybp (Wolfe et al., 1989): The closure of the carpel, and the germination of pollen on a sporophytic organ (stigmatic area) rather than in or on the micropyle of a mature seed (as in most gymnosperms).
It is generally thought that angiosperm pollen with multiple apertures first evolved from monosulcate pollen during the Cretaceous angiosperm radiation. Therefore, polyaperturate (e.g. tricolpate) angiosperm pollen could not exist prior to the Cretaceous, right? The Crinopolles Group, however, experimented with various types of multiple apertures 100 million years before the Cretaceous radiation. In contrast to angiosperm pollen evolution in the Cretaceous, polyaperturate species rapidly became extinct, while monosulcate species survived. The monosulcate type (Monocrinipollis) evolved not from a single aperture (as in gymnosperms), but from the coalescence of multiple distal apertures - a compound aperture found today only in the monocots (See figures below).
 Calectasia
cyanea pollen (Xanthorrhoeaceae: Asparagales) and Nelumbo
lutea pollen (American lotus: Nelumbonaceae) may be tetrasulcate and trisulcate Crinopolles survivors,
respectively. The existence of tetrasulcate pollen can be explained if Crinopolles
pollen is included in the picture of basal angiosperm pollen evolution.
Calectasia
cyanea pollen (Xanthorrhoeaceae: Asparagales) and Nelumbo
lutea pollen (American lotus: Nelumbonaceae) may be tetrasulcate and trisulcate Crinopolles survivors,
respectively. The existence of tetrasulcate pollen can be explained if Crinopolles
pollen is included in the picture of basal angiosperm pollen evolution.
Left: Image from Chanda
et al. (1978).
Consequently, the following characteristics in combination are a key angiosperm
synapomorphy (i.e., unique to angiosperms): Compound monosulcus;
tectate-columellate-reticulate exine; non-laminated endexine (or lack of endexine).
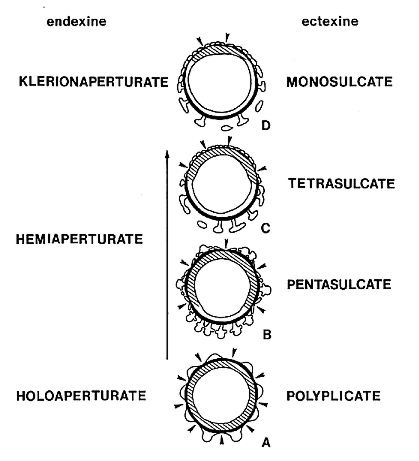
Above two figures from Cornet, 1989a.
Critical to understanding the significance of monosulcate pollen in Mesozoic palynofloras, only monosulcate angiosperm-like pollen flourished, and continued to evolve through the Norian, and Rhaetian. More familiar monocot-like morphotypes appeared in the Norian (e.g. Liliacidites and Retimonocolpites), while in the Rhaetian magnoliid-like morphotypes appeared.
Magnolia pollen looks like this (SEM and TEM images):
 |
Cornet was able to trace angiosperm-like pollen (specifically Cycadopites reticulatus) into Early Jurassic strata of the Newark Supergroup, eastern North America (Cornet and Traverse, 1975; Cornet, 1977; Cornet, 2000). Meaning: some angiosperm-like pollen producers survived the mass extinction event at the Triassic-Jurassic boundary. Nilsson (1958) reported C. reticulatus in the Rhaeto-Liassic coal flora of Sweden, indicating that this species migrated out of semiarid subtropical areas of the Newark Supergroup and into more equitable climatic zones to the north (Olsen and Kent, 2000).
The name Cycadopites is a formgenus, and has no taxonomic implication. It's use is predicated by rules established for botanical nomenclature.
Click on image below for a more detailed chronology of pollen morphotypes.
Resembling the diversification of angiosperm pollen in the Early Cretaceous (cf. Lupia, 1999), a multiaperturate condition rapidly evolved, but not from a monosulcate precursor (Cornet, 1989a). Subsequent selection favored not just the monosulcate type, but also a reduction in the size of the reticulum and the height of columellae, until a fine reticulum sat almost directly upon the underlying nexine in latest Triassic and Early Jurassic angiosperm-like pollen (cf. Cornet, 1977).
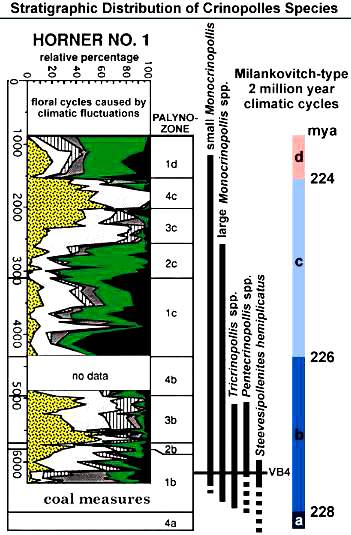
Data from Cornet (1994) and Cornet (1989).
The trend is cumulative through the Late Triassic, not linear with replacement: Pollen types with coarse to medium reticuli persist in small numbers, while types with very fine reticuli and short columellae appear very early in the sequence. In fact, Monocrinopollis microreticulatus is the most successful and widespread species in the Crinopolles Group, becoming the dominant angiosperm-like type in late Carnian strata of North Carolina, New Mexico, and Arizona (Cornet, 1989a).
 |
In other words, angiosperm-like pollen converged on the morphology of contemporaneous monosulcate pollen produced by the Bennettitales (extinct) and Ginkgoales (i.e., monosulcate, tectate-granular, imperforate: cf. Cornet and Traverse, 1975). Monosulcate Cycad pollen has a different internal exine structure. If this trend continued into the Jurassic, some angiosperm-like pollen would become indistinguishable from gymnosperm pollen using only optical and SEM techniques, accounting in part for the apparent palynological 'gap' in data for Jurassic angiosperms: Hence the confusion about tectate-granular magnoliid pollen being plesiomorphic (primitive) rather than convergent (e.g. Doyle, 1977; Foster & Price, 1981; Ward et al., 1989; see pollen comparison below).
Doyle (2005) corrects some of these long standing misconceptions about angiosperm pollen in Early evolution of angiosperm pollen as inferred from molecular and morphological analyses.
Abstract. Phylogenetic analyses of molecular and morphological data, revised in this study, shed light on longstanding controversies on the early evolution of angiosperm pollen. Although relationships between angiosperms and other seed plants remain uncertain, the robust rooting of the angiosperms among the ‘‘ANITA’’ groups (Amborella, Nymphaeales, Illiciales, Trimenia, and Austrobaileya) clarifies their original pollen morphology. Contrary to the view that the first angiosperms had boat-shaped monosulcate pollen with granular or atectate exine structure, the ANITA rooting implies that globose monosulcate pollen with more or less columellar structure and a continuous tectum was ancestral and a foveolate-reticulate tectum arose soon after. The oldest recognized Cretaceous angiosperm pollen may represent the latter grade of evolution. Structure described as granular evolved independently from columellar within Nymphaeales, Magnoliales, and Laurales. In Magnoliales, columellar Myristicaceae and Magnoliaceae diverge below Degeneria, Galbulimima, Eupomatia, and Annonaceae, which shifted to granular structure. Granular monosulcate pollen was ancestral in Annonaceae but gave rise to columellar monosulcates and permanent tetrads. In Laurales, reduction and granularization culminated in the fragile exines of Lauraceae. Although absence of a distinctly staining endexine in Magnoliales has been considered evidence that the laminated endexine of gymnosperms was lost before the origin of angiosperms, presence of a thin endexine now appears to be ancestral. These results refute the view that granular structure supports a relationship between angiosperms and Gnetales, Bennettitales, and Pentoxylon. Relationships with groups with alveolar exines (e.g., Caytonia, glossopterids) and/or reticulate-columellar Triassic Crinopolles pollen now seem equally likely. Summary
| Deduced from Extant Angiosperms | Crinopolles Group
Trend Through Late Triassic |
Crinopolles Examples from Cornet (1989) and Cornet (1986 unpublished) |
| Tectate-granular pollen
appears last in molecular- derived trees |
Tectate-granular microperforate and imperforate pollen appears last at top of Triassic |  |
| Boat-shaped pollen appears last | Boat-shaped Magnolia-like pollen appears at top of Triassic | 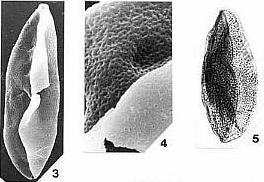 |
| Endexine thins or disappears in more derived ANHITA taxa (e.g. Chloranthaceae) | Endexine disappears (footlayer homogenous) in derived Crinopolles taxa (Norian-Rhaetian) | 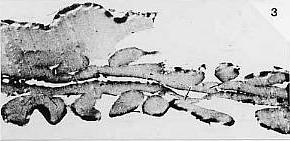  |
| Tricolpate pollen appears soon after ANHITA group evolution | Trisulcate pollen (Tricrinopollis) grades into Tricolpate pollen in oldest Crinopolles members | 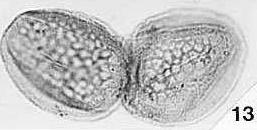 |
| Tri/hexacolpate pollen appears very early | Polycolpopollis (tricolpate) and Cornetipollis appear very early | 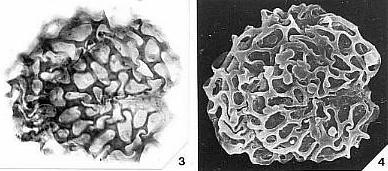 |
| Tectal performations appear very early | A fine reticulum rapidly appears and joins heads of clavae in oldest Crinopolles pollen |  |
| Footlayer and endexine present in oldest ANHITA Group | Footlayer and endexine are present in oldest Crinopolles types |
|
| Earliest tectum continuous | Earliest tectum continuous in polyplicate types |   |
| Earliest angiosperm pollen is globose | Earliest Crinopolles pollen ranges from elliptical to globose |    |
| Columellae appear very early | Columellae develop from free-standing clavae in least-derived Crinopolles taxa |  |
| Supra spinules appear very early | Supra gemmi and clavae appear very early |  |
| Earliest extant angiosperm pollen is monosulcate | Earliest Crinopolles taxa range from polyplicate to polysulcate to polycolpate to monosulcate |    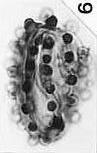 |
Pseudo-tricolpate Pollen
During the Mesozoic a pseudotricolpate or trisulcate condition arose in Eucommiidites
pollen, which was shown to have a gymnospermous-type laminated endexine by Doyle et
al. (1975). Subsequently, Pedersen et al. (1989; Friis and Pedersen,
1996) recovered Eucommiidites from an Upper Cretaceous (Cenomanian) pollen organ,
Erdtmanitheca texensis, and from the micropyles of a gymnospermous seed, Erdtmanispermum
balticum.
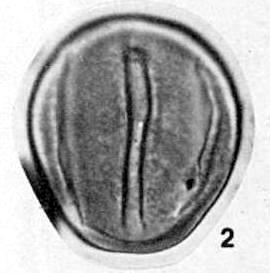
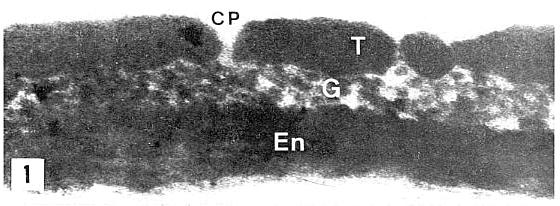
Images from Doyle et al. (1975, Plates 1 and 2: CP = corrosion pit; T = tectum; G = intratectal granules; En = endexine with laminations).
The tectate-granular semiperforate condition of Eucommiidites spp. indicates that multiple apertures and a magnoliid-like intragranular condition, by themselves or together, are not reliable indicators of an angiospermous affinity. The presence of tall columellae and a large reticulum may still be an angiosperm synapomorphy (i.e., unique to angiosperms). The absence of a gymnospermous-type laminated endexine (alternating light and dark laminae) in the Crinopolles Group and the absence (inferred loss) of an endexine in Norian-Rhaetian angiosperm-like pollen (see TEM cross section above and Liliacidites below), however, does imply a non-gymnospermous origin (Cornet, 1989a).
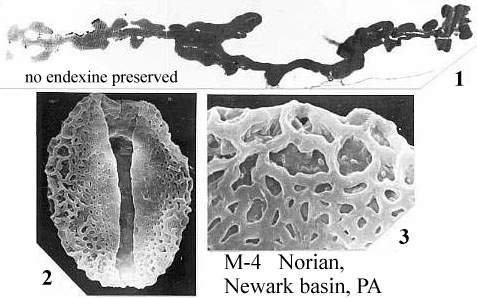 |
Zonasulculate pollen has been reported in several families of magnoliid angiosperms (Annonaceae, Atherospermataceae, Eupomatiaceae, and Nymphaeaceae) and monocots (Arecaceae, Araceae, Dasypogonaceae, Laxmanniaceae, and Rapateaceae). In the youngest Triassic strata of the Newark Supergroup (just below the Triassic-Jurassic boundary) similar pollen (Chasmatosporites spp.) is found as monads rather than as tetrads, which is characteristic of pre-Wintera pollen (e.g. Shrankipollis: Doyle et al., 1990). Chasmatosporites sp. N (figs. 3-4 below) and Cycadopites reticulatus (Magnolia-like pollen) were initially found by Nilsson (1958) in the Rhaeto-Liassic coals of Sweden. Whether these pollen types are magnoliid or monocotyledonid (cf. Monstera), their presence at the end of the Triassic supports the phylogenetic tree above, which shows the roots of basal angiosperms diversifying in the Late Triassic rather than the Early Cretaceous.
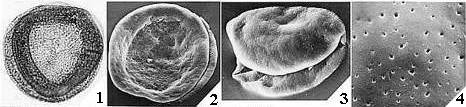
Tectate-columellate-perforate zonasulculate pollen with distal poroid thinning from palynoflorule JB2 (latest Rhaetian) of the Newark basin, NJ-PA (Cornet, 1977: Ph.D. thesis: Pl. 5, figs. 3-8). 1-2 Chasmatosporites sp. R; 3-4 Chasmatosporites sp. N.
Doyle (1977) notes: "Interestingly, a few Zone I monosulcates (Liliacidites sp. A: Plate I, 6) show exine sculpture patterns (coarsely reticulate on most of the grain surface, but fine at the ends of the grain and sulcus margins) common today among monocots but unknown in magnoliid dicots [unknown in gymnosperm monosulcate pollen also], suggesting that the basic split between monocots and dicots had already occurred (Doyle, 1973)." The Late Triassic pollen record for the Newark Supergroup shows magnoliid pollen characteristics evolving from pollen that combines monocot and dicot characteristics (e.g. the Crinopolles Group, before the basic split).
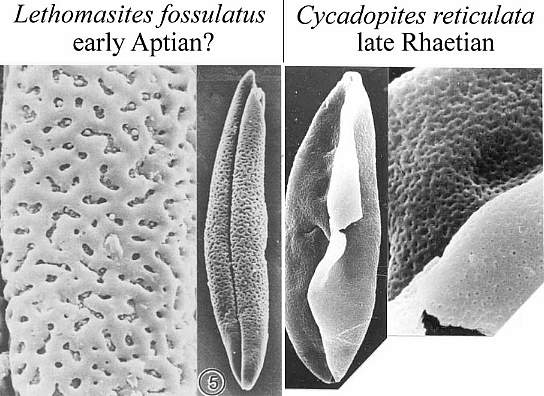
Images on left from Ward et al., 1989; specimen on right picked by Cornet from palynoflorule JB2, Newark basin, PA (SEM images by Jim and Audrey Walker, 1975).
 Left: Jim and Audrey Walker, University of
Massachusetts, Nov. 1976.
Left: Jim and Audrey Walker, University of
Massachusetts, Nov. 1976.
The distinctive psilate distal ends and large size (>60 mu) of the Early Cretaceous Lethomasites are also characteristics of late Rhaetian tectate-granular perforate and imperforate monosulcates of the Newark Supergroup, North America. Large pollen (> 90 mu) such as Cycadopites schlischii also implies insect involvement in dispersal, due to higher weight and reduced air buoyancy. This pollen type was first recognized by Cornet in the latest Triassic JB2 palynoflorule from the Exeter Village excavation (Triassic-Jurassic boundary in the Jacksonville syncline) in the southern part of the Newark basin, PA (Monosulcate sp. X in his Ph.D. thesis), and later discovered by Fowell (Ph.D. thesis) in the latest Triassic Blomidon Fm. of the Fundy basin, New Brunswick and Nova Scotia, Canada (named by Fowell and Traverse, 1995).
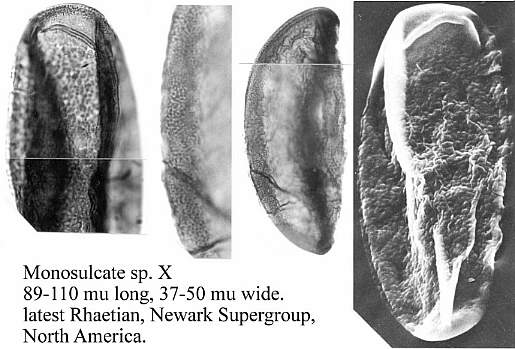
Another odd feature in common is that both the Cretaceous and Triassic magnoliid monosulcates contain examples where they are rolled up like a scroll. If the Aptian (Early Cretaceous) examples can be classified as angiosperm pollen, then so too can the Rhaetian (Late Triassic) examples. The TEM section below is the same grain illustrated as Cycadopites reticulatus above (TEM section by Jim and Audrey Walker, 1975).
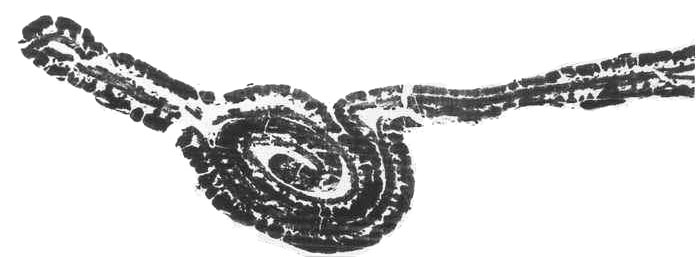
Empirically, this remarkable trend in exine reduction was later reversed in the Late Jurassic and Early Cretaceous. The diversity of pollen morphology and exine structure in primitive extant magnoliids implies that "granular" exines in the Magnoliales are probably derived, an implication supported by molecular data (Martin et al., 1993). Furthermore, the presence of magnoliid pollen characteristics in the latest Triassic and Early Jurassic indicates that the roots of angiospermy extend further back in time, a possibility also supported by molecular data (Chaw et al., 2000). Vasanthy et al. (1990) hypothesize that the "angiospermid" pollen characters of the Late Triassic were lost and reappeared in the Early Cretaceous. The fossil pollen evidence presented here supports that idea via convergent evolution, although more typical angiosperm-like pollen may have survived during the Jurassic in isolated populations (e.g. Clavatipollenites and Stellatopollis: Cornet and Habib, 1992). Why reduction occurred after a functional reticulate-columellate tricolpate existed in the early Carnian is a mystery.
|
If Cretaceous monosulcates and tricolpates are genetically derived from Triassic Crinopolles pollen, genes for multiple apertures may be suppressed and archived in monosulcate-producing magnoliid angiosperms (see evolutionary reversals). Geneticists may be able to resolve such genes: Four recent papers have characterized the transcription profile of pollen grains, showing striking differences between gene expression in pollen and other plant tissues. These studies increase the number of known pollen-expressed genes by as much as 50-fold and have identified many novel genes that are potentially pollen-specific (da Costa-Nunes and Grossniklaus, 2003). The question that should be asked is, what caused a gradual selection for the monosulcate condition and exine reduction in the Triassic? Extinction is not the answer for the loss of "angiospermid" pollen characters, because this trend is gradual over a period of 27 million years, and has been traced to magnoliid-like pollen in the Jurassic.
Beetles - Incidental Pollination
If Hymenoptera are largely responsible for rapid diversification of angiosperms in the Albian-Turonian and the return of multiaperturate pollen (specifically tricolpate and tricolpate-derived pollen), then selection by another group of insects might be responsible for the dominance of monosulcate pollen over multiaperturate pollen in the Late Triassic and Jurassic. Study the graphs on insect diversification through the Phanerozoic above. The one group of insects that shows diversification beginning in the Ladinian (late Middle Triassic), and continuing through the Mesozoic and Cenozoic is the Coleoptera.
Below: Evolution of pre-Cretaceous angiosperm-like pollen compared to beetle diversification.
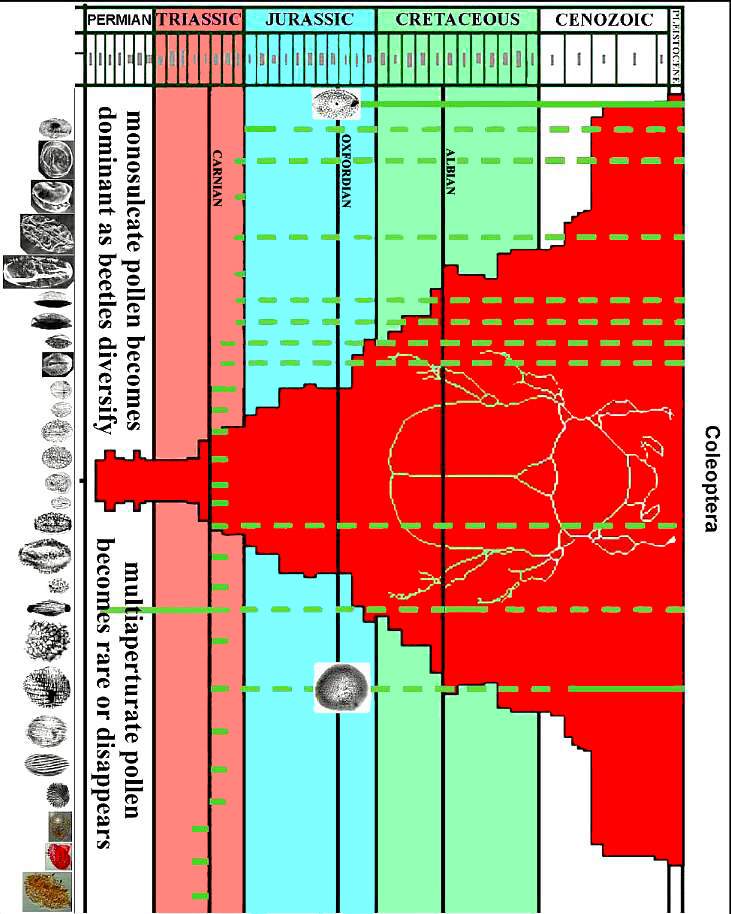
Dashed lines indicate morphotypes (e.g. Liliacidites, Retimonocolpites, Stellatopollis, and Multimarginites) that continue to the present (although produced by different species). Solid lines (or pictures) within a dashed interval indicate reported occurrences of this morphotype in younger strata. Implication: convergence of pollen morphology between gymnosperms and angiosperms was a product of selection, possibly by beetle pollinators. Above figure modified from Labandeira, Eble, and Santa Fe Institute, 2001.
The trend towards loss of columellae and tectal performations, and restriction of apertures to one would be a function of selection, but what kind of selection and why? Perhaps beetles that fed on pollen reacted to taste, and chemicals involved in gametophytic and sporophytic incompatibility might make pollen with multiple apertures and a porous exine (i.e., more chemical leakage) more distasteful or interfere with digestion (obviously not so for the Hymenoptera, which seek nectar instead of pollen). Thus, there may have been a selection by herbaceous beetles for plants that produced monosulcate pollen. For example, beetle pollinated species of extant Magnoliaceae tend to produce imperforate to microperforate monosulcate pollen. The suspected pollinators of the Degeneriaceae are also beetles (Miller, 1989). Evidence for Sanmiguelia and Archaestrobilus (late Carnian - early Norian) indicates that beetles may have been their primary pollinator (distinctive bite marks, transported anther fragments on carpel, glands or food bodies, etc.). The pollen of both is psilate-imperforate and monosulcate (Cornet, 1989a; 1996)! And if this explanation is correct (i.e., simple monosulcate pollen is not necessarily basic or primitive; Doyle, 2005), then early (basal) angiosperms may have had much competition from the Cheirolepidaceae, Bennettitales, Cycadales, and Gingoales (i.e., Mesozoic gymnosperms) through beetle foraging and incidental pollination (i.e., not dependable), accounting in part for their lack of success until flower-dedicated bee pollinators (Hymenoptera) evolved in the Cretaceous. For example, basal angiosperms make up only 1-2% of floral communities during the Triassic, Jurassic, and earliest Cretaceous (see Cornet, 1989a; 1992; Brenner, 1996). And it was the extinction of the Cheirolepidaceae in the Cenomanian that preceeded a major increase in floral diversity among angiosperms in the Turonian (Lupia et al., 2000; cf. Crepet, 2000).
From "Brian Farrell meets the beetles" in the Harvard Gazette (Gehrman, 2002):
"Studying beetles' evolutionary biology, for instance, gave Farrell insight to a question that had puzzled scientists for decades - possibly centuries: Why are there so many beetles? In a paper published in the July 1998 issue of Science, Farrell concluded that it's because "every time they colonized plants, particularly flowering plants, their diversity leapt up by several orders of magnitude" as the plants developed defenses to combat them. Today, more than half of beetles are plant feeders - and they have a huge advantage over their predatory and scavenger brethren simply because plants are so plentiful. "So the reason why there are so many beetles collectively" Farrell says, "is because of repeated origins of herbivory.""
From "Brian Farrell in Bugdom" in the Harvard Magazine (Shaw, 2003):
"Farrell published his exegesis of Haldan's dilemma - "Inordinate Fondness' Explained: Why are there so many beetles?" - in Science in 1998. He showed that the number of species exploded whenever a beetle made the jump to feeding on angiosperms (flowering plants, which come in many species), from feeding on gymnosperms (conifers, cycads, ginkgoes, and the like, with their more limited numbers of species).
"But he also showed that these insect communities are profoundly old and had been feeding on plants long before the angiosperm explosion, the period when flowering plants suddenly appeared. Over time, as gymnosperm species diversity worldwide dwindled due to the adaptive advantages of angiosperms, the vast majority of beetle groups did not make the switch to flowering plants. "There is a profound implication here," says Farrell. "The age of these insect groups suggests that there surely must have been opportunities for better host plants available over time. But," he says, "they stayed on the same host even as it dwindled in abundance, which implies to me that there are genetic limits to the abilities of species [of beetles] to shift among resources." "If those plants go extinct," says Farrell, "so will those beetles."
"On the other side, every time a beetle group did make the shift over to angiosperm feeding, "diversity went up between a hundred- and a thousandfold, contributing several hundred thousand new species to the planet." Angiosperm feeders now make up half of all beetle groups. All of the major herbivorous groups of insects (bees, ants, wasps, butterflies, moths) follow a similar pattern. They first made the shift from carnivory by feeding on primitive plant groups, says Farrell, "mostly on the cones of either cycads or conifers," which are the most protein-rich parts of plants, and therefore, from a nutritional standpoint, most like the insect bodies from which these former carnivores weaned themselves over the course of evolutionary time. Then a few groups shifted even further onto angiosperms and speciated rapidly."
Based on Farrell's concept of beetle speciation being driven more by angiosperm speciation than by gymnosperm speciation, most of the exponential increase in beetle species between the Ladinian (Middle Triassic) and Aalenian (Middle Jurassic), and again between the Kimmeridgian (Late Jurassic) and Cenomanian (early Late Cretaceous: see beetle diagram above) is not due to gymnosperm diversification. It is due to angiosperm diversification (compare with bootstrap replicates chart above, which shows times of genetic innovation and speciation among angiosperms). Note also that there is a drop in species diversity in the Turonian-Santonian (Late Cretaceous) following the demise of the most successful group of Mesozoic gymnosperms - the Cheirolepidaceae, something that Farrell predicted would happen: "But they stayed on the same host even as it dwindled in abundance, which implies to me that there are genetic limits to the abilities of [beetle] species to shift among resources." "If those plants go extinct," says Farrell, "so will those beetles."
Cheirolepid Conifer Adaptation
The Circumpolles Group of pollen (listed below) is distinct from the time of its first appearance in the Middle Triassic. "The pollen of gymnosperms possibly related to the Cheirolepidaceae or their ancestors first appears in Laurasia in the Ladinian (Middle Triassic) under the names Duplicisporites, Praecirculina, Paracirculina, Partitisporites, and Spiritisporites. These taxa continue through the Carnian (Scheuring, 1970; Brugman, 1983), and become extinct in the early Norian in western Europe (Germanic and Alpine facies: Brugman, 1983). Looking westward across Laurasia, these taxa can be found in the rift basins of the Newark Supergroup, eastern North America, but only Duplicisporites has so far been recorded in the lower Chinle Formation of Arizona and New Mexico (Dunay and Fisher, 1974; Cornet, 1977; Litwin et al., 1991; Cornet, 1993).
"Granuloperculatopollis rudis and Classopollis meyerianus first appear in the middle Norian of Europe, while Classopollis torosus first appears in the Rhaetian. Granuloperculatopollis disappears at the end of the Triassic. In the Newark Supergroup C. meyerianus has been found in the Chatham-Richmond-Taylorsville Palynofloral Zone (middle Carnian) of the Deep River Basin, NC (e.g., Schultz and Hope, 1973), while C. torosus first appears in the basal Manassas-Upper Passaic Palynofloral Zone, which is the youngest Triassic palynozone of the Newark Supergroup (Cornet, 1977; Cornet and Olsen, 1985; Cornet, 1993). By the Rhaetian Classopollis becomes the dominant conifer pollen type worldwide, sometime making up 90% or more of local palynofloras near the Rhaeto-Liassic boundary in Europe (Danzé-Corsin and Laveine, 1963; Venkatachala and Goçzan, 1964; Fisher, 1972; Herngreen and DeBoer, 1974). Overall, these pollen taxa, which are related based on morphology and exine structure/sculpture, show high diversity beginning in the Middle Triassic, with those taxa producing Classopollis adapting better than their relatives, and eventually replacing them in the Rhaeto-Liassic." (Cornet and Waanders, 2006).It is important to recognize that both pollen groups: the Circumpolles Group (affinity Cheirolepidaceae) and the Crinopolles Group (affinity basal angiosperms) coevolved in the Ladinian (i.e., in the same region but not necessarily in the same habitats), and both diversified in tropical and subtropical areas through the Late Triassic, with Classopollis pollen becoming the most-derived Circumpolles pollen type, and reticulate-columellate monosulcate pollen (e.g., Monocrinopolles microreticulatus) becoming the most-derived Crinopolles pollen type. It was also during the Ladinian that beetles began their diversification (see diagram above). Whereas the Crinopolles Group always remained a minor component of Late Triassic and Early Jurassic palynofloras, the Circumpolles Group started out as a minor component, but became the dominant element of most lower latitude Rhaeto-Liassic palynofloras.
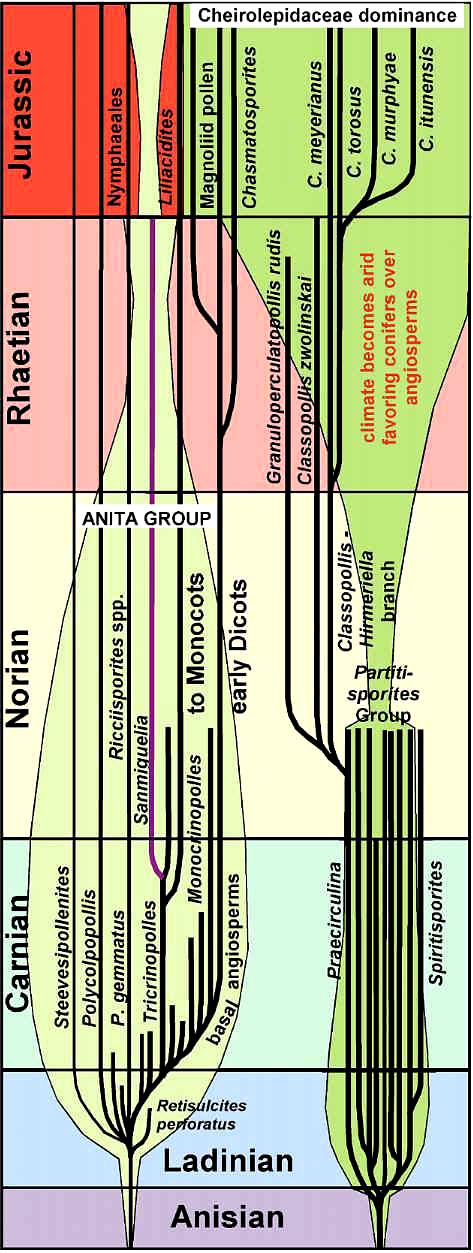
Above: Two types of diagrams are superimposed to show 1) branching nature of Crinopolles and Circumpolles pollen groups in the Middle and Late Triassic, and 2) the relative or comparable abundance of each group in the areas where they evolved (hour-glass-shaped diagrams). Whereas angiosperm-like pollen decreased in abundance as wet tropical and semiarid subtropical climates of Pangaea deteriorated through the Late Triassic, the conifers that produced circumpolles pollen were already adapted for aridity. Even though the Partitisporites Group became extinct in the early Norian (near the time of the Manicougan asteroid impact), Classopollis evolved in southwestern Europe just before that extinction, enabling the Cheirolepidaceae to diversify and fill vacated ecological niches to become the dominant pollen type during the Jurassic and Early Cretaceous. This reciprocal relationship deserves additional research, because it might shed verifiable data on why basal angiosperms became subordinate to the Cheirlolepidaceae until the Albian. Phylogenetic trees for Crinopolles and Circumpolles groups constructed from data in Cornet (1989a) and Brugman (1983).
The Cheirolepidaceae evolved from arborescent ancestors around the Tethys Sea (the oldest area of greatest circumpolles pollen diversity), while the first angiosperms were probably small shrubs, vines, and paleoherbs, restricted to humid if not wet habitats. The Cheirolepidaceae became adapted to arid, semiarid, and physiologically arid (i.e., salty) habitats, while basal angiosperms were limited in distribution by their physiological requirement for moist and possibly dark understory habitats. As tropical and subtropical regions of Pangaea became more arid during the Late Triassic, angiosperm-like pollen diversity dropped (i.e., only found in perennial lake deposits, but not in ephemeral or playa lake deposits). By comparison, cheirolepid pollen became more abundant, and is found in fluvial facies associated with brackish to saline (i.e., gypsum- and halite-producing) playa lake deposits (e.g. Moroccan and Fundy Triassic basins).
"Based on the characteristics of the ovulate cones, their structure, and the dehiscence of the seed bearing scales, Jung (1968) suggests that members of the Cheirolepidaceae are transitional between the Voltziales and the Taxodiaceae. However, cuticular preparations indicate that the ovules were surrounded by highly complex cone scales, which suggests that the group may be derived with respect to reproductive biology (Watson, 1988). This suggestion is supported by the complex structure of the Classopollis pollen, which may have served in a specialized pollen-recognition system similar to those of flowering plants (Chaloner, 1976; Watson, 1988)." (Stewart and Rothwell, 1993: 430).
It was not until the Circumpolles Group disappeared in the Cenomanian that angiosperm diversity increased rapidly (Lupia et al., 2000). This inverse relationship began in the Late Triassic with the rise of the Cheirolepidaceae to world dominance. It implies some sort of mutual exclusion or competition - not necessarily for the same habitats as much as for the same insect pollinators, i.e., beetles, during the Jurassic and earliest Cretaceous. What basal angiosperms couldn't do by increasing their diversity, cheirolepid conifers did by increasing their abundance and stature (height), and by developing a symbiotic relationship with herbivorous dinosaurs, who spread their protected seeds in warm moist piles of dung, which became populated by beetle larvae. And it was not until the Hymenoptera (bees) came to the rescue and support of basal angiosperms that we see a shift in dominance between cheirolepid conifers and angiosperms in the Early Cretaceous. It was also during the Early Cretaceous that we see a shift in the average size of herbivorous dinosaurs from tall tree crown eaters to short herb and shrub eaters, who could help disperse the seeds of low-stature basal angiosperms and provide manure as the sauropods did for the Cheirolepidaceae in the Jurassic (Bakker, 1978).
The earliest known conifer that produced Classopollis pollen is Hirmeriella meunsteri, from the Rhaeto-Liassic of Germany (Jung, 1968). It had microphyllous leaves and lived in coastal environments (i.e., xerophytic trees adapted to physiological drought). Near the end of their world dominance in the Albian, diversity of coastal cheirolepids had increased, producing strange horsetail-like vegetation described under Frenelopsis and Pseudofrenelopsis. The habit of these conifers is envisioned as small shrubs (see F. ramosissima below), similar to the habit envisioned for Early Cretaceous coastal angiosperms (Doyle and Hickey, 1977). For example, the Albian coastal swamp facies along the Paluxy River in northeastern Glen Rose, Texas, are dominated by both Frenelopsis and Pseudofrenelopsis (Cheirolepidaceae), along with a diverse assemblage of microphyllous conifers, most of which were probably related to the Cheirolepidaceae. The palynoflora is strongly dominated by Classopollis spp. (70+%), the characteristic pollen of the Cheirolepidaceae. More information with images of fossils. Retallack and Dilcher (1981) suggest that angiosperms first arose in coastal environments, which if true means that they would have had to compete directly with the dominant gymnosperms in those environments, most of which were chirolepid conifers.
Above: A) Brachyphyllum scottii from the basal Hettangian, Newark basin, NJ; B) Ovuliferous scale of B. scottii (abaxial and adaxial sides, showing seed which outgrew and ruptured its ovuliferous scale pouch) from the basal Hettangian, Culpeper basin, VA; C) Hirmeriella meunsteri reconstruction (Stewart and Rothwell, 1993: from Fig. 29.14); D) H. meunsteri ovuliferous scale (Jung, 1969) from Rhaeto-Liassic; E) Hirmeriella kendalliae (Harris, 1979); F) Frenelopsis hohenggeri (Fontaine, 1894; Perkins et al., 1979: Pl. 4) from Albian Glen Rose Fm., TX; G) Frenelopsis varians (Fontaine, 1894; Perkins et al., 1979: Pl. 3) from Albian Glen Rose Fm., TX; H) Frenelopsis ramosissima (Stewart and Rothwell, 1993: from Fig. 29.14) from Early Cretaceous; I) reconstruction of Frenelopsis ramosissima (Stewart and Rothwell, 1993: from Fig. 29.14).
Competition with Cheirolepidaceous Conifers
The
Cheirolepidaceae are now thought to be most closely related to the extant Araucariaceae,
which also have their seeds  enclosed within a scale-bract complex (a concept normally reserved
for angio sperms, which means cloaked seeds).
The discovery of an Early Jurassic (Sinemurian) cheirolepid
conifer in central Pangaea ("Conanthus hystrixipinus")
that produced small simple flowers with perianth, ovuliferous scales with unusually
elongate sterile lobes (modified for wind dispersal), a pair of hollow
stigma-style-like organs (modified sterile lobes), and delayed ovule/ovary development
until after fertilization, led to the recognition that some beetle-pollinated Cretaceous
flowers reported in the literature may not belong to angiosperms (see What about Lequeria?). Beetle elytra are common in the same
sedimentary layers containing "Conanthus", supporting the idea that
this Magnolia-like gymnosperm was insect pollinated, something that the
tectate-columellate pollen (Classopollis) of the Cheirolepidaceae has suggested
to palynologists for decades. Implications: Basal angiosperms had some serious
competition with arborescent entomophyllous gymnosperms in the Jurassic.
enclosed within a scale-bract complex (a concept normally reserved
for angio sperms, which means cloaked seeds).
The discovery of an Early Jurassic (Sinemurian) cheirolepid
conifer in central Pangaea ("Conanthus hystrixipinus")
that produced small simple flowers with perianth, ovuliferous scales with unusually
elongate sterile lobes (modified for wind dispersal), a pair of hollow
stigma-style-like organs (modified sterile lobes), and delayed ovule/ovary development
until after fertilization, led to the recognition that some beetle-pollinated Cretaceous
flowers reported in the literature may not belong to angiosperms (see What about Lequeria?). Beetle elytra are common in the same
sedimentary layers containing "Conanthus", supporting the idea that
this Magnolia-like gymnosperm was insect pollinated, something that the
tectate-columellate pollen (Classopollis) of the Cheirolepidaceae has suggested
to palynologists for decades. Implications: Basal angiosperms had some serious
competition with arborescent entomophyllous gymnosperms in the Jurassic.
Above left from Vasanthy et al. (2004: Plate IV): Classopollis minor Pocock & Jansonius 1961. 1. Distal view, paral membrane detached exposing the edges of endo-annulus; note the sub-equatorial furrow (white arrow). x 3600. (SEM by S.A.J. Pocock). Classopollis harrisii Muir & Van Kon. Middle-Jurassic: Rhaeto-Liassic. 2. Note the columellar complexity (C), faint lamellations within the nexine and the complex tectum (T). x l3100 (by J.Medus).
Below: Vegetative branches and a portion of an isolated female cone containing mature winged seeds of the Early Jurassic "Conanthus hystrixipinus". This conifer shows some of the longest leaves of any currently-known cheirolepid conifer. Amazingly, palynomorphs are better preserved in this red shale than in associated gray and black shales, perhaps due to the high copper content of the sediments (visible as greenish gray auras around the leafy shoots). http://www.sunstar-solutions.com/sunstar/Conanthus/conanthus.htm Scale in centimeters.

Below left: Male cone attached to leafy shoot (arrow), next to a female cone at flower stage. Note that both reproductive structures are of similar size and shape at the same time in development, providing comparable targets for foraging beetles. Below right: Classopollis torosus (28.5 mu in diameter), the pollen type found in the male cone of "Conanthus".
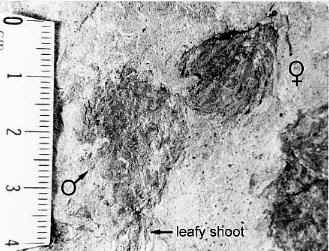
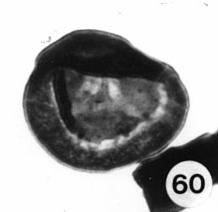
Below: Two flowers of "Conanthus hystrixipinus" prior to complete opening and fertilization, and before significant elongation and enlargement to produce a fertilized seed cone. Outer bracts elongated first to produce a perianth, while inner fertile ovuliferous scales completed development after fertilization. We know this because the cone closed up as it enlarged (see pictures below), and seeds-seed scales did not reach maturity until the cone doubled in size. Scale in centimeters. For supporting evidence, see Text-figure 6 in Cornet and McDonald (unpublished manuscript).
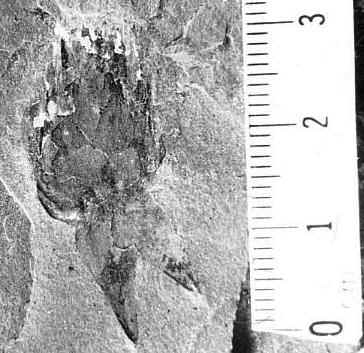

Below: Graph showing male and female floral development of "Conanthus hystrixipinus" from immature to seed dispersal stage, and the male cones (black squares) from which mature Classopollis pollen was extracted.
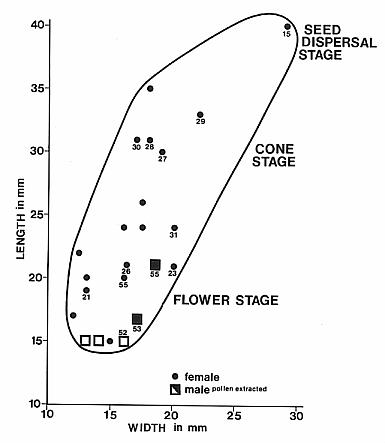
The closest analog to external floral morphology among extant angiosperms can be found in the Asteraceae, in which basal bracts reflex to expose a floral head of densely-packed unicarpellate flowers. Beetles commonly forage on and pollinate aster species. In the case for "Conanthus", the gynoecium would be comprised of densely-packed immature ovuliferous scales and their subtending bracts (analogous to aster unicarpellate flowers). It is speculated that the unique stigma-style-like ovuliferous scale lobes of "Conanthus" developed first so that foraging beetles could transfer pollen from male cones. Compare floral development with Xanthium spinosum (below left: Asteraceae), and with form of Carduus tenuiflorus (below right: Asteraceae) with stigmas-styles projecting above bracts.


The above image use granted by © Br. Alfred Brousseau, Saint Mary's College (left) and Steve Shoenig 2005 (right).
Below: Strobilus following fertilization at flower stage (i.e., gynoecium enlarges and closes up as seeds develop), but before ovuliferous scales reach maturity. Note resemblance to immature Magnolia strobilus. Scale in centimeters. For supporting evidence, see Text-figure 6 in Cornet and McDonald (unpublished manuscript).


Below: Seed dispersal stage, showing ovuliferous scale lobes sticking out beyond strobilus, waiting to be caught by the wind. There is an uncanny resemblance to the basic stages of Magnolia flower, fruit, and seed dispersal, suggesting that the Magnoliaceae may have converged upon a reproductive plan or strategy that already existed among gymnosperms in the Mesozoic. Could beetles have been the driving force behind such convergence?

Below: Dispersed (mature) ovuliferous scale with paired stigmatic lobes (arrows: modified sterile scale lobes - round with hollow centers that communicated with ovary and contained a resin-like material, also found in ovary) and single seed, which has fallen out of pouch (ovary) and lies below the ovuliferous scale. Scale in centimeters.
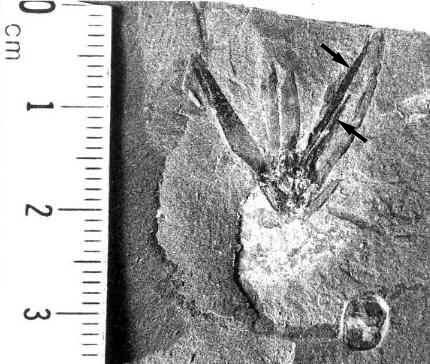
AD: Enjoy the real success with 70-457
braindumps and cisco 1z0-051
training programs and latest ccnp
syllabus. Also prepare for next level with quality 640-916 and answers of HITACHI training.
[12.24.14-17]
Bruce Cornet currently lives near El Paso, TX, and teaches physical geology, historical geology, and evolution.
This page was created on 3 June 2002, and last updated on 11/24/2014.